Page 135 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 135
4.8 CARBENE AND ALKENE ANALOGS 115
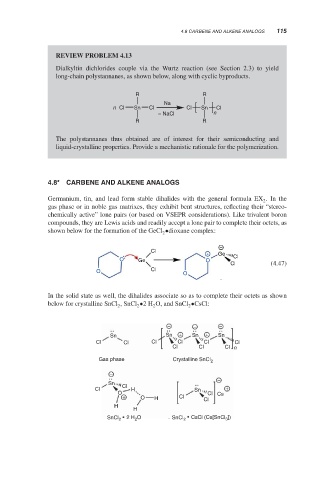
REVIEW PROBLEM 4.13
Dialkyltin dichlorides couple via the Wurtz reaction (see Section 2.3) to yield
long-chain polystannanes, as shown below, along with cyclic byproducts.
R R
Na
n Cl Sn Cl Cl Sn Cl
− NaCl n
R R
The polystannanes thus obtained are of interest for their semiconducting and
liquid-crystalline properties. Provide a mechanistic rationale for the polymerization.
4.8* CARBENE AND ALKENE ANALOGS
Germanium, tin, and lead form stable dihalides with the general formula EX .Inthe
2
gas phase or in noble gas matrixes, they exhibit bent structures, reflecting their “stereo-
chemically active” lone pairs (or based on VSEPR considerations). Like trivalent boron
compounds, they are Lewis acids and readily accept a lone pair to complete their octets, as
shown below for the formation of the GeCl •dioxane complex:
2
−
Cl
+ Ge Cl
O Ge O
Cl (4.47)
O Cl O
In the solid state as well, the dihalides associate so as to complete their octets as shown
below for crystalline SnCl , SnCl •2H O, and SnCl •CsCl:
2
2
2
2
− − −
Sn Sn + Sn + Sn
Cl Cl Cl Cl Cl Cl
Cl Cl Cl n
Gas phase Crystalline SnCl 2
−
−
Sn
Cl Cl H Sn +
O Cl Cs
+ O H Cl Cl
H
H
2 H O SnCl CsCl (Cs[SnCl ])
SnCl 2 2 2 3