Page 136 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 136
GROUP 14 ELEMENTS
116
The divalent Ge and Sn halides are moderately reactive and insert into or add to various
reactive bonds, as in the following cycloaddition:
I I
Ge Ge (4.48)
I
I
Divalent organocompounds can be synthesized from the corresponding group 14 dihalides
and organolithiums. With highly sterically hindered aryllithiums, one can obtain relatively
stable monomeric group 14 diaryl derivatives. By analogy with carbene, these compounds
are known as silylenes (Si), germylenes (Ge), stannylenes (Sn), and plumbylenes (Pb):
Ar
EX 2 + 2 ArLi E + 2 LiX
Ar
)
X = Cl, Br, I, N(SiMe 3 2
(4.49)
A A
Ar = A = Me, Et, cyclohexyl, i-Pr, Ph, Mes
B = H, Me, i-Pr, t-Bu
B
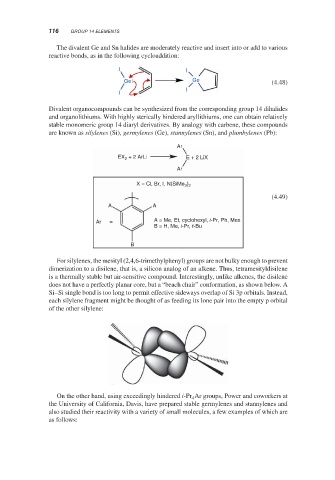
For silylenes, the mesityl (2,4,6-trimethylphenyl) groups are not bulky enough to prevent
dimerization to a disilene, that is, a silicon analog of an alkene. Thus, tetramesityldisilene
is a thermally stable but air-sensitive compound. Interestingly, unlike alkenes, the disilene
does not have a perfectly planar core, but a “beach chair” conformation, as shown below. A
Si–Si single bond is too long to permit effective sideways overlap of Si 3p orbitals. Instead,
each silylene fragment might be thought of as feeding its lone pair into the empty p orbital
of the other silylene:
On the other hand, using exceedingly hindered i-Pr Ar groups, Power and coworkers at
4
the University of California, Davis, have prepared stable germylenes and stannylenes and
also studied their reactivity with a variety of small molecules, a few examples of which are
as follows: