Page 132 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 132
GROUP 14 ELEMENTS
112
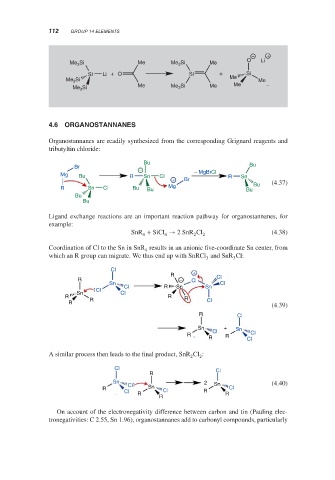
− +
O Li
Me Si Me Me Si Me
3
3
Si Li + O Si + Si
Si Me
Me 3 Me Me
Si Me Me 3 Si Me
Me 3
4.6 ORGANOSTANNANES
Organostannanes are readily synthesized from the corresponding Grignard reagents and
tributyltin chloride:
Bu
Br Bu
−
Mg Bu R Sn Cl − MgBrCl R Sn
+ Br (4.37)
R Sn Cl Bu Bu Mg Bu Bu
Bu
Bu
Ligand exchange reactions are an important reaction pathway for organostannanes, for
example:
SnR + SiCl → 2 SnR Cl (4.38)
4 4 2 2
Coordination of Cl to the Sn in SnR results in an anionic five-coordinate Sn center, from
4
which an R group can migrate. We thus end up with SnRCl and SnR Cl:
3
3
Cl
R + Cl
R − Cl
Sn Cl
Cl R Sn Sn
Cl
Sn Cl
R R
R R Cl
R
(4.39)
R Cl
Sn + Sn
R Cl R Cl
R Cl
A similar process then leads to the final product, SnR Cl :
2 2
Cl Cl
R
Sn 2 (4.40)
Cl Sn Sn
R Cl Cl
Cl R R R
R
On account of the electronegativity difference between carbon and tin (Pauling elec-
tronegativities: C 2.55, Sn 1.96), organostannanes add to carbonyl compounds, particularly