Page 127 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 127
4.4 THE -SILICON EFFECT: ALLYLSILANES 107
1
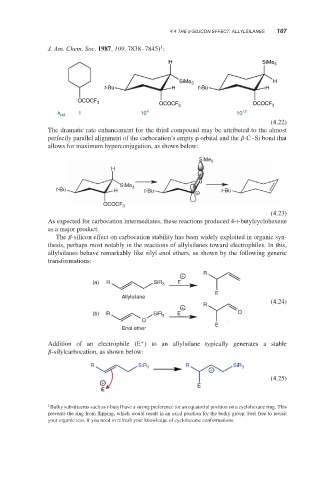
J. Am. Chem. Soc. 1987, 109, 7838–7845) :
H SiMe 3
H
SiMe 3
t-Bu H t-Bu H
OCOCF 3
OCOCF 3 OCOCF 3
k rel 1 10 4 10 12
(4.22)
The dramatic rate enhancement for the third compound may be attributed to the almost
perfectly parallel alignment of the carbocation’s empty p orbital and the -C–Si bond that
allows for maximum hyperconjugation, as shown below:
SiMe 3
H
SiMe 3
t-Bu H t-Bu + t-Bu
OCOCF 3
(4.23)
As expected for carbocation intermediates, these reactions produced 4-t-butylcyclohexene
as a major product.
The -silicon effect on carbocation stability has been widely exploited in organic syn-
thesis, perhaps most notably in the reactions of allylsilanes toward electrophiles. In this,
allylsilanes behave remarkably like silyl enol ethers, as shown by the following generic
transformations:
+ R
(a) R SiR 3 E
E
Allylsilane
(4.24)
+ R
(b) R SiR 3 E O
O
E
Enol ether
+
Addition of an electrophile (E ) to an allylsilane typically generates a stable
-silylcarbocation, as shown below:
R SiR 3 R SiR 3
+
(4.25)
+ E
E
1 Bulky substituents such as t-butyl have a strong preference for an equatorial position on a cyclohexane ring. This
prevents the ring from flipping, which would result in an axial position for the bulky group. Feel free to revisit
your organic text, if you need to refresh your knowledge of cyclohexane conformations.