Page 122 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 122
GROUP 14 ELEMENTS
102
Silicon tetrafluoride also reacts vigorously with water, but the reaction may be
described as an incomplete hydrolysis:
2− +
2SiF + 4H O → Si(OH) + SiF 6 + 2H + 2HF
2
4
4
Explain the different behaviors exhibited by the different group 14 halides.
Note: Si(OH) is a simplified formula; the actual product is a mixture of polymeric
4
silanols, homologs of (HO) Si–O–Si(OH) .
3 3
−
Reagents of the form R SiY, where Y is a nucleophile, are an important class of
3
−
organosilicon reagents. They are primarily used for addition of Y to carbonyl groups.
Space will not permit a proper discussion here, but a good example of such a reagent is
trimethylsilyl cyanide (Me SiCN), which we discussed in Section 1.14. Another excel-
3
lent example is the Ruppert–Prakash reagent (Me SiCF ), discussed below in Review
3
3
problem 4.5.
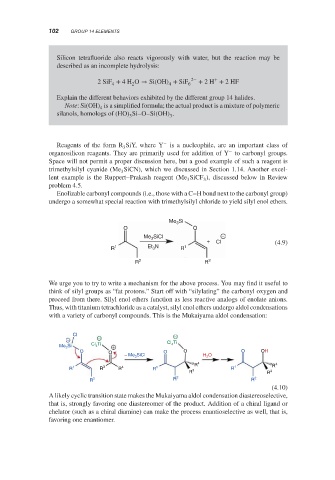
Enolizable carbonyl compounds (i.e., those with a C–H bond next to the carbonyl group)
undergo a somewhat special reaction with trimethylsilyl chloride to yield silyl enol ethers.
Si
Me 3
O O O
Me 3 SiCl −
+ Cl (4.9)
R 1 Et 3 N R 1
R 2 R 2
We urge you to try to write a mechanism for the above process. You may find it useful to
think of silyl groups as “fat protons.” Start off with “silylating” the carbonyl oxygen and
proceed from there. Silyl enol ethers function as less reactive analogs of enolate anions.
Thus, with titanium tetrachloride as a catalyst, silyl enol ethers undergo aldol condensations
with a variety of carbonyl compounds. This is the Mukaiyama aldol condensation:
Cl −
− −
Cl 4 Ti Cl Ti
4
Me 3 Si +
O O O O O O OH
− Me 3 SiCl H 2 O
R 4 R 4
R 1 R 3 R 4 R 1 R 1
R 3 R 3
R 2 R 2 R 2
(4.10)
A likely cyclic transition state makes the Mukaiyama aldol condensation diastereoselective,
that is, strongly favoring one diastereomer of the product. Addition of a chiral ligand or
chelator (such as a chiral diamine) can make the process enantioselective as well, that is,
favoring one enantiomer.