Page 119 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 119
4.1 SILYL PROTECTING GROUPS 99
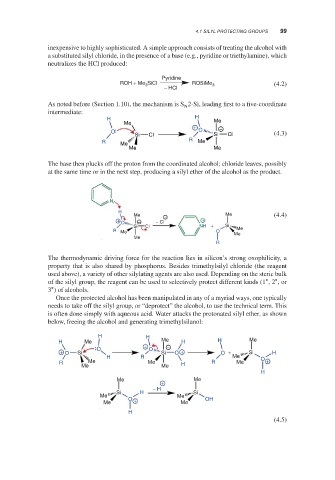
inexpensive to highly sophisticated. A simple approach consists of treating the alcohol with
a substituted silyl chloride, in the presence of a base (e.g., pyridine or triethylamine), which
neutralizes the HCl produced:
Pyridine
ROH + Me 3 SiCl ROSiMe 3 (4.2)
− HCl
As noted before (Section 1.10), the mechanism is S 2-Si, leading first to a five-coordinate
N
intermediate:
H H Me
Me
+ −
O O
Si Cl Si Cl (4.3)
R
R Me Me
Me Me
The base then plucks off the proton from the coordinated alcohol; chloride leaves, possibly
at the same time or in the next step, producing a silyl ether of the alcohol as the product.
N
H
Me − Me (4.4)
+ O − − Cl +
Si Cl NH + Si
R Me O Me
Me
Me
R
The thermodynamic driving force for the reaction lies in silicon’s strong oxophilicity, a
property that is also shared by phosphorus. Besides trimethylsilyl chloride (the reagent
used above), a variety of other silylating agents are also used. Depending on the steric bulk
∘
∘
of the silyl group, the reagent can be used to selectively protect different kinds (1 ,2 ,or
∘
3 ) of alcohols.
Once the protected alcohol has been manipulated in any of a myriad ways, one typically
needs to take off the silyl group, or “deprotect” the alcohol, to use the technical term. This
is often done simply with aqueous acid. Water attacks the protonated silyl ether, as shown
below, freeing the alcohol and generating trimethylsilanol:
H H
H Me + Me H H Me
+ O Si O O Si − O + O + Si H
H R Me O
R Me Me H R Me +
Me Me
H
Me Me
+
− H
Si H Si
Me Me
O + OH
Me Me
H
(4.5)