Page 123 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 123
4.2 A CASE STUDY: PETERSON OLEFINATION 103
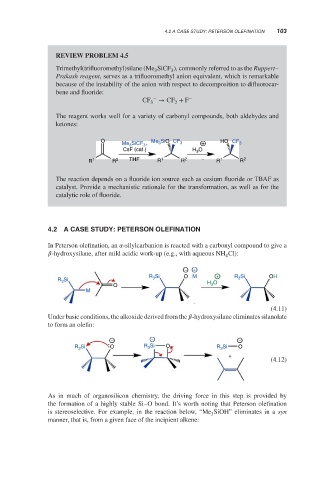
REVIEW PROBLEM 4.5
Trimethyl(trifluoromethyl)silane (Me SiCF ), commonly referred to as the Ruppert–
3 3
Prakash reagent, serves as a trifluoromethyl anion equivalent, which is remarkable
because of the instability of the anion with respect to decomposition to difluorocar-
bene and fluoride:
− −
CF → CF + F
3 2
The reagent works well for a variety of carbonyl compounds, both aldehydes and
ketones:
O Me SiO CF HO CF
Me 3 SiCF 3 , 3 3 + 3
CsF (cat.) H O
3
R 1 R 2 THF R 1 R 2 R 1 R 2
The reaction depends on a fluoride ion source such as cesium fluoride or TBAF as
catalyst. Provide a mechanistic rationale for the transformation, as well as for the
catalytic role of fluoride.
4.2 A CASE STUDY: PETERSON OLEFINATION
In Peterson olefination, an -silylcarbanion is reacted with a carbonyl compound to give a
-hydroxysilane, after mild acidic work-up (e.g., with aqueous NH Cl):
4
− +
Si O M + R Si OH
R 3
R Si H O 3
3
O 3
M
(4.11)
Under basic conditions, the alkoxide derived from the -hydroxysilane eliminates silanolate
to form an olefin:
− − −
R Si O R 3 Si O R 3 Si O
3
+
(4.12)
As in much of organosilicon chemistry, the driving force in this step is provided by
the formation of a highly stable Si–O bond. It’s worth noting that Peterson olefination
is stereoselective. For example, in the reaction below, “Me SiOH” eliminates in a syn
3
manner, that is, from a given face of the incipient alkene: