Page 124 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 124
GROUP 14 ELEMENTS
104
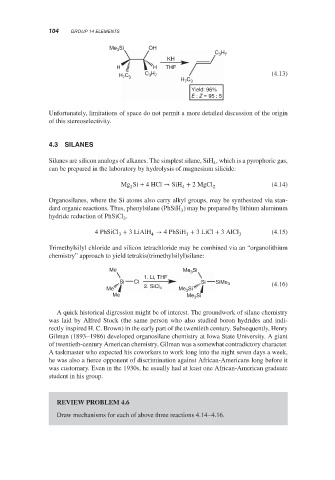
Me Si OH
3
C 3 H 7
KH
H H THF
C
3 7
H 7 3 C H (4.13)
H 7 C 3
Yield: 96%
E : Z = 95 : 5
Unfortunately, limitations of space do not permit a more detailed discussion of the origin
of this stereoselectivity.
4.3 SILANES
Silanes are silicon analogs of alkanes. The simplest silane, SiH , which is a pyrophoric gas,
4
can be prepared in the laboratory by hydrolysis of magnesium silicide:
Mg Si + 4HCl → SiH + 2MgCl 2 (4.14)
2
4
Organosilanes, where the Si atoms also carry alkyl groups, may be synthesized via stan-
dard organic reactions. Thus, phenylsilane (PhSiH ) may be prepared by lithium aluminum
3
hydride reduction of PhSiCl .
3
4 PhSiCl + 3 LiAlH → 4 PhSiH + 3LiCl + 3AlCl 3 (4.15)
3
3
4
Trimethylsilyl chloride and silicon tetrachloride may be combined via an “organolithium
chemistry” approach to yield tetrakis(trimethylsilyl)silane:
Me Me Si
3
1. Li, THF
Si Cl Si SiMe 3 (4.16)
Me 2. SiCl 4 Me 3 Si
Me Me 3 Si
A quick historical digression might be of interest. The groundwork of silane chemistry
was laid by Alfred Stock (the same person who also studied boron hydrides and indi-
rectly inspired H. C. Brown) in the early part of the twentieth century. Subsequently, Henry
Gilman (1893–1986) developed organosilane chemistry at Iowa State University. A giant
of twentieth-century American chemistry, Gilman was a somewhat contradictory character.
A taskmaster who expected his coworkers to work long into the night seven days a week,
he was also a fierce opponent of discrimination against African-Americans long before it
was customary. Even in the 1930s, he usually had at least one African-American graduate
student in his group.
REVIEW PROBLEM 4.6
Draw mechanisms for each of above three reactions 4.14–4.16.