Page 128 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 128
GROUP 14 ELEMENTS
108
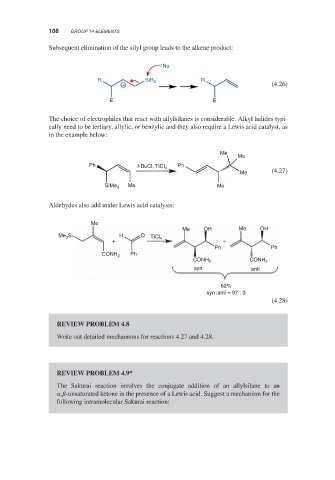
Subsequent elimination of the silyl group leads to the alkene product:
Nu
R SiR 3 R
+ (4.26)
E E
The choice of electrophiles that react with allylsilanes is considerable. Alkyl halides typi-
cally need to be tertiary, allylic, or benzylic and they also require a Lewis acid catalyst, as
in the example below:
Me
Me
Ph Ph
t-BuCI, TiCI 4
Me (4.27)
SiMe 3 Me Me
Aldehydes also add under Lewis acid catalysis:
Me
Me OH Me OH
Me Si H O TiCl 4
3
+ +
Ph Ph
Ph
CONH 2
CONH 2 CONH 2
syn anti
62%
syn:anti = 97 : 3
(4.28)
REVIEW PROBLEM 4.8
Write out detailed mechanisms for reactions 4.27 and 4.28.
REVIEW PROBLEM 4.9*
The Sakurai reaction involves the conjugate addition of an allylsilane to an
, -unsaturated ketone in the presence of a Lewis acid. Suggest a mechanism for the
following intramolecular Sakurai reaction: