Page 130 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 130
GROUP 14 ELEMENTS
110
of 346 ± 3 kcal/mol, some 26 kcal/mol “higher” than that of silane. The solution pK of
a
tris(trimethylsilyl)silane has been found to be 29.4 (Korogodsky, G., et al. Organometallics
2002, 21, 3157–3161), which corresponds to a somewhat higher acidity than triphenyl-
methane (pK 31.3).
a
REVIEW PROBLEM 4.10
Tris(trimethylsilyl)silane is much more acidic than tris(trimethylsilyl)methane
(pK = 36.8) and triphenylsilane (pK = 35.1). Can you think of an explanation for
a
a
these relative acidities? Also, can you suggest a rationale for the high acidity of
trichlorosilane (recall that it can be deprotonated by triethylamine).
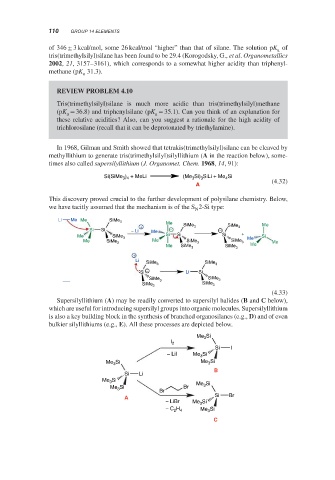
In 1968, Gilman and Smith showed that tetrakis(trimethylsilyl)silane can be cleaved by
methyllithium to generate tris(trimethylsilyl)silyllithium (A in the reaction below), some-
times also called supersilyllithium (J. Organomet. Chem. 1968, 14, 91):
Si(SiMe ) + MeLi (Me Si) SiLi + Me Si
4
3
3 4
3
(4.32)
A
This discovery proved crucial to the further development of polysilane chemistry. Below,
we have tacitly assumed that the mechanism is of the S 2-Si type:
N
Li Me Me SiMe 3
Me SiMe
+ 3 SiMe 3 Me
Si Si − Li Me − −
Me SiMe Si Si Si + Si
3 Me
Me SiMe 3 Me SiMe 3 SiMe 3 Me
Me SiMe 3 SiMe 3 Me
+
Li
SiMe 3 SiMe 3
−
Si Li Si
SiMe SiMe
3 3
SiMe SiMe 3
3
(4.33)
Supersilyllithium (A) may be readily converted to supersilyl halides (B and C below),
which are useful for introducing supersilyl groups into organic molecules. Supersilyllithium
is also a key building block in the synthesis of branched organosilanes (e.g., D) and of even
bulkier silyllithiums (e.g., E). All these processes are depicted below.
Me 3 Si
I 2
Si I
− Lil Me Si
3
Me 3 Si Me Si
3
B
Si Li
Me Si Si
3
Si Br Me 3
Me 3
Br
A Si Br
− LiBr Me 3 Si
− C H Me 3 Si
2 4
C