Page 133 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 133
4.7 POLYSTANNANES 113
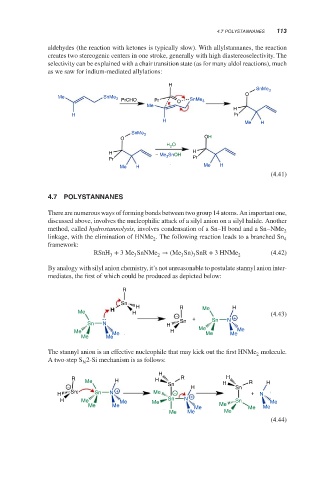
aldehydes (the reaction with ketones is typically slow). With allylstannanes, the reaction
creates two stereogenic centers in one stroke, generally with high diastereoselectivity. The
selectivity can be explained with a chair transition state (as for many aldol reactions), much
as we saw for indium-mediated allylations:
H
SnMe 3
O
Me SnMe 3 PrCHO Pr SnMe 3
Me O
H
H Pr
H
Me H
SnMe 3
OH
O
O
H 2
H
H SnOH
− Me 3 Pr
Pr
Me H Me H
(4.41)
4.7 POLYSTANNANES
There are numerous ways of forming bonds between two group 14 atoms. An important one,
discussed above, involves the nucleophilic attack of a silyl anion on a silyl halide. Another
method, called hydrostannolysis, involves condensation of a Sn–H bond and a Sn–NMe
2
linkage, with the elimination of HNMe . The following reaction leads to a branched Sn
2 4
framework:
RSnH + 3Me SnNMe → (Me Sn) SnR + 3 HNMe (4.42)
3 3 2 3 3 2
By analogy with silyl anion chemistry, it’s not unreasonable to postulate stannyl anion inter-
mediates, the first of which could be produced as depicted below:
R
Sn
H R Me H
Me H H
− + (4.43)
Sn + Sn N
Sn N H
Me Me H Me Me Me Me
Me Me
The stannyl anion is an effective nucleophile that may kick out the first HNMe molecule.
2
Atwo-stepS 2-Si mechanism is as follows:
N
H
R H R H
Me H H R H
− Sn H Sn
Sn Sn N + Me
H − + + N
H Me Me Me Sn N Sn Me
Me Me Me Me Me Me
Me Me Me
(4.44)