Page 131 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 131
4.5 SILYL ANIONS 111
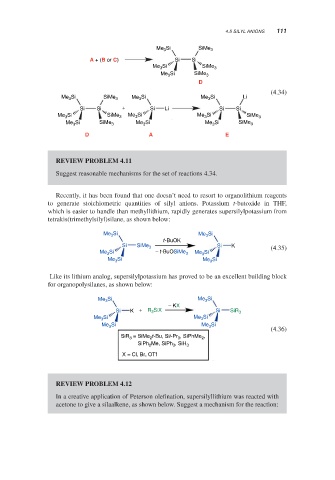
Me 3 Si SiMe 3
A + (B or C) Si Si
Me 3 Si SiMe 3
Si
Me 3 SiMe 3
D
(4.34)
Me 3 Si SiMe 3 Me Si Me 3 Si Li
3
Si Si + Si Li Si Si
Me 3 Si SiMe 3 Me 3 Si Me 3 Si SiMe 3
Me 3 Si SiMe 3 Me Si Me 3 Si SiMe 3
3
D A E
REVIEW PROBLEM 4.11
Suggest reasonable mechanisms for the set of reactions 4.34.
Recently, it has been found that one doesn’t need to resort to organolithium reagents
to generate stoichiometric quantities of silyl anions. Potassium t-butoxide in THF,
which is easier to handle than methyllithium, rapidly generates supersilylpotassium from
tetrakis(trimethylsilyl)silane, as shown below:
Me 3 Si Me 3 Si
t-BuOK
Si SiMe 3 Si K (4.35)
Me 3 Si − t-BuOSiMe 3 Me 3 Si
Me Si Me Si
3
3
Like its lithium analog, supersilylpotassium has proved to be an excellent building block
for organopolysilanes, as shown below:
Me 3 Si Me 3 Si
− KX
Si K + R 3 SiX Si SiR 3
Me Si Me 3 Si
3
Me 3 Si Me Si
3
(4.36)
SiR 3 = SiMe 2 t-Bu, Sii-Pr 3 , SiPhMe 2 ,
SiPh Me, SiPh , SiH 3
2
3
X = Cl, Br, OTf
REVIEW PROBLEM 4.12
In a creative application of Peterson olefination, supersilyllithium was reacted with
acetone to give a silaalkene, as shown below. Suggest a mechanism for the reaction: