Page 134 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 134
GROUP 14 ELEMENTS
114
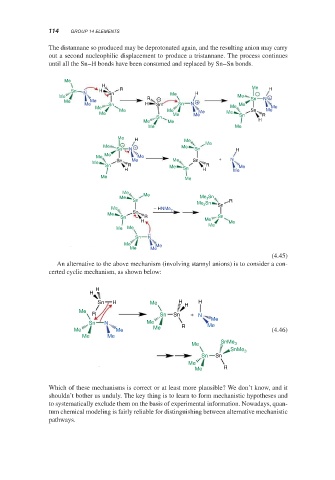
The distannane so produced may be deprotonated again, and the resulting anion may carry
out a second nucleophilic displacement to produce a tristannane. The process continues
until all the Sn–H bonds have been consumed and replaced by Sn–Sn bonds.
Me
H Me
Sn N H Sn R H H
Me R Me Me Sn − N +
Me Me − +
Me Sn H Sn Sn N Me
Me Me Me Me Sn Me Me
Me Me Me Me Me Sn R
Sn
Me Me H
Me Me
Me
H Me
Me − + Me Me
Sn N Sn H
Me
Me Me
Sn Me Me Sn + N
Me
Sn R Me R Me
H Sn H Me
Me
Me
Me
Me Me Me 3 Sn
Sn R
Me Sn Sn
3
Me − HNMe 2
Me Sn
Sn R Me Sn
H Me
Me
Me Me
Sn N
Me Me
Me Me
(4.45)
An alternative to the above mechanism (involving stannyl anions) is to consider a con-
certed cyclic mechanism, as shown below:
H
H
Sn H Me H H
H
Me
R Sn Sn + N
Sn N Me Me
Me R Me
Me Me (4.46)
Me Me
SnMe
Me 3
SnMe 3
Sn Sn
Me
Me R
Which of these mechanisms is correct or at least more plausible? We don’t know, and it
shouldn’t bother us unduly. The key thing is to learn to form mechanistic hypotheses and
to systematically exclude them on the basis of experimental information. Nowadays, quan-
tum chemical modeling is fairly reliable for distinguishing between alternative mechanistic
pathways.