Page 125 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 125
4.3 SILANES 105
Silicon being more electropositive than hydrogen (Pauling electronegativities: Si 1.90,
H 2.20), silanes with Si–H bonds can reduce a variety of functional groups such as carbonyl
compounds, imines, and even alcohols by transferring a hydride anion. Triethylsilane is a
common reagent, and typical reaction conditions also include either a Brønsted or a Lewis
acid, as shown below for the reduction of a ketone:
H
O O
Et 3 SiH, CF 3 COOH,
(4.17)
R 1 R 2 CH Cl 2 R 1 H
2
R 2
The first step of the mechanism is expected to be a protonation:
O
O
F C O
3
F C −
3
R 1 O
H + (4.18)
O R 1 H
R 2 O
+
R 2
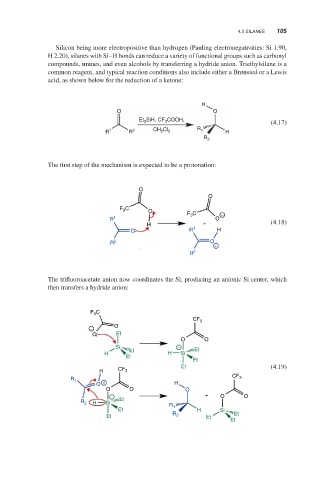
The trifluoroacetate anion now coordinates the Si, producing an anionic Si center, which
then transfers a hydride anion:
F 3 C
CF 3
− O
O Et
O O
Si −
Et Et
H H Si
Et
Et
H CF 3 Et (4.19)
CF 3
R 1 +
O H
O O O
− Et + O O
R 2 H Si
R 1
Et H Si
Et R 2 Et Et
Et