Page 120 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 120
GROUP 14 ELEMENTS
100
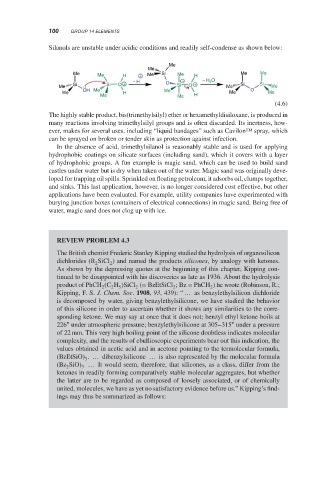
Silanols are unstable under acidic conditions and readily self-condense as shown below:
Me
Me
Me Me H + Me Si Me H Me Me
Si Si O + − H O Si − O + − H 2 O Si Si
Me Me Me
Me OH Me H Me Me O Me
Me Me H
(4.6)
The highly stable product, bis(trimethylsilyl) ether or hexamethyldisiloxane, is produced in
many reactions involving trimethylsilyl groups and is often discarded. Its inertness, how-
ever, makes for several uses, including “liquid bandages” such as Cavilon™ spray, which
can be sprayed on broken or tender skin as protection against infection.
In the absence of acid, trimethylsilanol is reasonably stable and is used for applying
hydrophobic coatings on silicate surfaces (including sand), which it covers with a layer
of hydrophobic groups. A fun example is magic sand, which can be used to build sand
castles under water but is dry when taken out of the water. Magic sand was originally deve-
loped for trapping oil spills. Sprinkled on floating petroleum, it adsorbs oil, clumps together,
and sinks. This last application, however, is no longer considered cost effective, but other
applications have been evaluated. For example, utility companies have experimented with
burying junction boxes (containers of electrical connections) in magic sand. Being free of
water, magic sand does not clog up with ice.
REVIEW PROBLEM 4.3
The British chemist Frederic Stanley Kipping studied the hydrolysis of organosilicon
dichlorides (R SiCl ) and named the products silicones, by analogy with ketones.
2
2
As shown by the depressing quotes at the beginning of this chapter, Kipping con-
tinued to be disappointed with his discoveries as late as 1936. About the hydrolysis
product of PhCH (C H )SiCl (= BzEtSiCl ;Bz = PhCH ) he wrote (Robinson, R.;
2 2 5 2 2 2
Kipping, F. S. J. Chem. Soc. 1908, 93, 439): “ … as benzylethylsilicon dichloride
is decomposed by water, giving benzylethylsilicone, we have studied the behavior
of this silicone in order to ascertain whether it shows any similarities to the corre-
sponding ketone. We may say at once that it does not; benzyl ethyl ketone boils at
∘
∘
226 under atmospheric pressure; benzylethylsilicone at 305–315 under a pressure
of 22 mm. This very high boiling point of the silicone doubtless indicates molecular
complexity, and the results of ebullioscopic experiments bear out this indication, the
values obtained in acetic acid and in acetone pointing to the termolecular formula,
(BzEtSiO) . … dibenzylsilicone … is also represented by the molecular formula
3
(Bz SiO) … It would seem, therefore, that silicones, as a class, differ from the
2
3
ketones in readily forming comparatively stable molecular aggregates, but whether
the latter are to be regarded as composed of loosely associated, or of chemically
united, molecules, we have as yet no satisfactory evidence before us.” Kipping’s find-
ings may thus be summarized as follows: