Page 117 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 117
GROUP 14 ELEMENTS 97
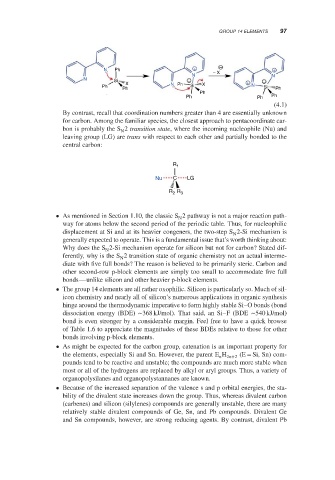
N Ph + − +
N − X N
N Si −
X N Ph X + −
Ph Si N Si
Ph Ph
Ph
Ph Ph Ph
(4.1)
By contrast, recall that coordination numbers greater than 4 are essentially unknown
for carbon. Among the familiar species, the closest approach to pentacoordinate car-
bon is probably the S 2 transition state, where the incoming nucleophile (Nu) and
N
leaving group (LG) are trans with respect to each other and partially bonded to the
central carbon:
R 1
Nu C LG
R 2 R 3
• As mentioned in Section 1.10, the classic S 2 pathway is not a major reaction path-
N
way for atoms below the second period of the periodic table. Thus, for nucleophilic
displacement at Si and at its heavier congeners, the two-step S 2-Si mechanism is
N
generally expected to operate. This is a fundamental issue that’s worth thinking about:
Why does the S 2-Si mechanism operate for silicon but not for carbon? Stated dif-
N
ferently, why is the S 2 transition state of organic chemistry not an actual interme-
N
diate with five full bonds? The reason is believed to be primarily steric. Carbon and
other second-row p-block elements are simply too small to accommodate five full
bonds—unlike silicon and other heavier p-block elements.
• The group 14 elements are all rather oxophilic. Silicon is particularly so. Much of sil-
icon chemistry and nearly all of silicon’s numerous applications in organic synthesis
hinge around the thermodynamic imperative to form highly stable Si–O bonds (bond
dissociation energy (BDE) ∼368 kJ/mol). That said, an Si–F (BDE ∼540 kJ/mol)
bond is even stronger by a considerable margin. Feel free to have a quick browse
of Table 1.6 to appreciate the magnitudes of these BDEs relative to those for other
bonds involving p-block elements.
• As might be expected for the carbon group, catenation is an important property for
the elements, especially Si and Sn. However, the parent E H 2n+2 (E = Si, Sn) com-
n
pounds tend to be reactive and unstable; the compounds are much more stable when
most or all of the hydrogens are replaced by alkyl or aryl groups. Thus, a variety of
organopolysilanes and organopolystannanes are known.
• Because of the increased separation of the valence s and p orbital energies, the sta-
bility of the divalent state increases down the group. Thus, whereas divalent carbon
(carbenes) and silicon (silylenes) compounds are generally unstable, there are many
relatively stable divalent compounds of Ge, Sn, and Pb compounds. Divalent Ge
and Sn compounds, however, are strong reducing agents. By contrast, divalent Pb