Page 112 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 112
GROUP 13 ELEMENTS
92
O
O
OTf
−
− OTf
O NMe 2 +
O NMe 2
H
DMF H
(3.52)
O
H 2 O
O O
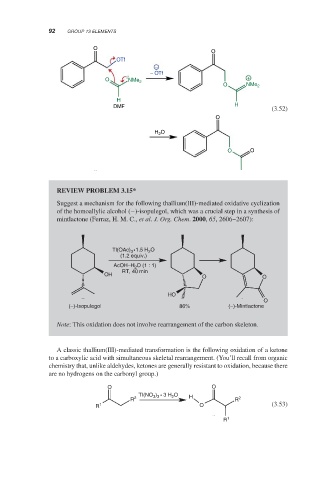
REVIEW PROBLEM 3.15*
Suggest a mechanism for the following thallium(III)-mediated oxidative cyclization
of the homoallylic alcohol (−)-isopulegol, which was a crucial step in a synthesis of
mintlactone (Ferraz, H. M. C., et al. J. Org. Chem. 2000, 65, 2606–2607):
Tl(OAc) •1.5 H O
2
3
(1.2 equiv.)
AcOH–H 2 O (1 : 1)
RT, 40 min
OH
O O
HO
O
(−)-Isopulegol 86% (−)-Mintlactone
Note: This oxidation does not involve rearrangement of the carbon skeleton.
A classic thallium(III)-mediated transformation is the following oxidation of a ketone
to a carboxylic acid with simultaneous skeletal rearrangement. (You’ll recall from organic
chemistry that, unlike aldehydes, ketones are generally resistant to oxidation, because there
are no hydrogens on the carbonyl group.)
O O
TI(NO 3 ) 3 • 3 H 2 O H
R 2 R 2
R 1 O (3.53)
R 1