Page 111 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 111
3.8 THALLIUM REAGENTS 91
REVIEW PROBLEM 3.14*
The compound TlI exists but it does not contain trivalent thallium. Can you
3
suggest an explanation? (Hint: What might be another reasonable valence state
for Tl?)
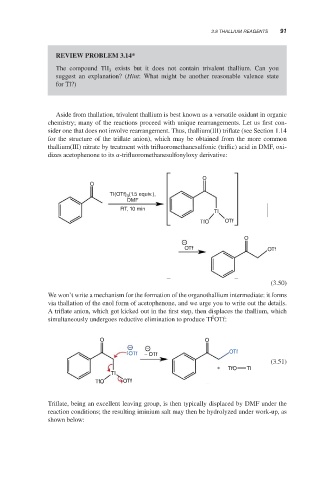
Aside from thallation, trivalent thallium is best known as a versatile oxidant in organic
chemistry; many of the reactions proceed with unique rearrangements. Let us first con-
sider one that does not involve rearrangement. Thus, thallium(III) triflate (see Section 1.14
for the structure of the triflate anion), which may be obtained from the more common
thallium(III) nitrate by treatment with trifluoromethanesulfonic (triflic) acid in DMF, oxi-
dizes acetophenone to its -trifluoromethanesulfonyloxy derivative:
O
O
(1.5 equiv.),
TI(OTf) 3
DMF
RT, 10 min
TI
TfO OTf
O
−
OTf OTf
(3.50)
We won’t write a mechanism for the formation of the organothallium intermediate: it forms
via thallation of the enol form of acetophenone, and we urge you to write out the details.
A triflate anion, which got kicked out in the first step, then displaces the thallium, which
I
simultaneously undergoes reductive elimination to produce Tl OTf:
O O
− −
OTf − OTf OTf
(3.51)
+ TfO TI
TI
TfO OTf
Triflate, being an excellent leaving group, is then typically displaced by DMF under the
reaction conditions; the resulting iminium salt may then be hydrolyzed under work-up, as
shown below: