Page 113 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 113
3.8 THALLIUM REAGENTS 93
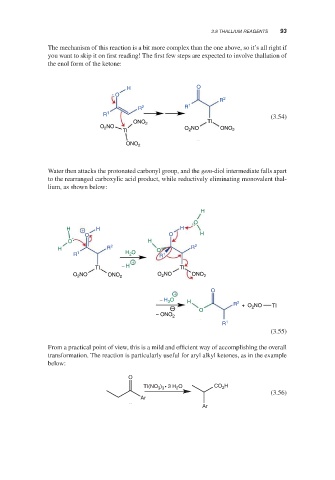
The mechanism of this reaction is a bit more complex than the one above, so it’s all right if
you want to skip it on first reading! The first few steps are expected to involve thallation of
the enol form of the ketone:
H O
O
R 2
R 2 R 1
R 1
(3.54)
TI
ONO 2
NO
O 2 O 2 NO
TI ONO 2
ONO 2
Water then attacks the protonated carbonyl group, and the gem-diol intermediate falls apart
to the rearranged carboxylic acid product, while reductively eliminating monovalent thal-
lium, as shown below:
H
O
H + H H
O O H
O H
H R 2 O R 2
R 1 H 2 O R 1
+
TI − H TI
NO O 2 NO ONO
O 2 ONO 2 2
O
+
− H 3 O H 2
− O R + O 2 NO TI
− ONO 2
R 1
(3.55)
From a practical point of view, this is a mild and efficient way of accomplishing the overall
transformation. The reaction is particularly useful for aryl alkyl ketones, as in the example
below:
O
TI(NO ) • 3 H O CO 2 H
3 3
2
(3.56)
Ar
Ar