Page 109 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 109
3.8 THALLIUM REAGENTS 89
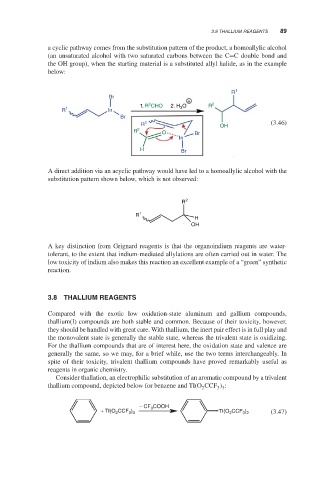
a cyclic pathway comes from the substitution pattern of the product, a homoallylic alcohol
(an unsaturated alcohol with two saturated carbons between the C=C double bond and
the OH group), when the starting material is a substituted allyl halide, as in the example
below:
R 1
Br
+
2
1. R CHO 2. H 3 O R 2
R 1 In
Br
R 1 OH (3.46)
R 2 O Br
In
H Br
A direct addition via an acyclic pathway would have led to a homoallylic alcohol with the
substitution pattern shown below, which is not observed:
R 2
R 1
H
OH
A key distinction from Grignard reagents is that the organoindium reagents are water-
tolerant, to the extent that indium-mediated allylations are often carried out in water. The
low toxicity of indium also makes this reaction an excellent example of a “green” synthetic
reaction.
3.8 THALLIUM REAGENTS
Compared with the exotic low oxidation-state aluminum and gallium compounds,
thallium(I) compounds are both stable and common. Because of their toxicity, however,
they should be handled with great care. With thallium, the inert pair effect is in full play and
the monovalent state is generally the stable state, whereas the trivalent state is oxidizing.
For the thallium compounds that are of interest here, the oxidation state and valence are
generally the same, so we may, for a brief while, use the two terms interchangeably. In
spite of their toxicity, trivalent thallium compounds have proved remarkably useful as
reagents in organic chemistry.
Consider thallation, an electrophilic substitution of an aromatic compound by a trivalent
thallium compound, depicted below for benzene and Tl(O CCF ) :
2 3 3
− CF 3 COOH
+ TI(O 2 CCF 3 ) 3 TI(O 2 CCF 3 ) 2 (3.47)