Page 108 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 108
GROUP 13 ELEMENTS
88
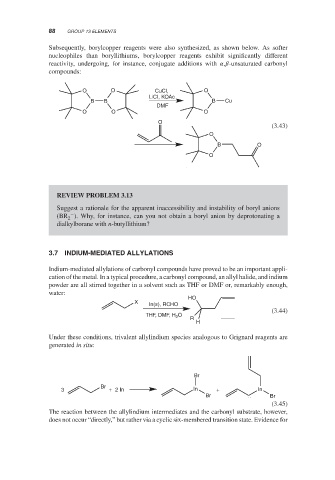
Subsequently, borylcopper reagents were also synthesized, as shown below. As softer
nucleophiles than boryllithiums, borylcopper reagents exhibit significantly different
reactivity, undergoing, for instance, conjugate additions with , -unsaturated carbonyl
compounds:
O O CuCI, O
LiCI, KOAc
B B B Cu
DMF
O O O
O
(3.43)
O
B O
O
REVIEW PROBLEM 3.13
Suggest a rationale for the apparent inaccessibility and instability of boryl anions
−
(BR ). Why, for instance, can you not obtain a boryl anion by deprotonating a
2
dialkylborane with n-butyllithium?
3.7 INDIUM-MEDIATED ALLYLATIONS
Indium-mediated allylations of carbonyl compounds have proved to be an important appli-
cation of the metal. In a typical procedure, a carbonyl compound, an allyl halide, and indium
powder are all stirred together in a solvent such as THF or DMF or, remarkably enough,
water:
HO
X In(s), RCHO
(3.44)
THF, DMF, H 2 O
R
H
Under these conditions, trivalent allylindium species analogous to Grignard reagents are
generated in situ:
Br
Br
3 + 2 In In + In
Br Br
(3.45)
The reaction between the allylindium intermediates and the carbonyl substrate, however,
does not occur “directly,” but rather via a cyclic six-membered transition state. Evidence for