Page 107 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 107
3.6 THE BORYL ANION 87
3.6 THE BORYL ANION
−
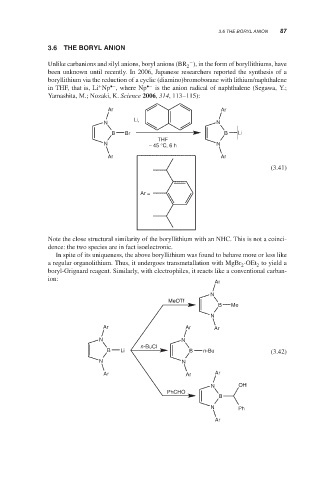
Unlike carbanions and silyl anions, boryl anions (BR ), in the form of boryllithiums, have
2
been unknown until recently. In 2006, Japanese researchers reported the synthesis of a
boryllithium via the reduction of a cyclic (diamino)bromoborane with lithium/naphthalene
+ •– •−
in THF, that is, Li Np , where Np is the anion radical of naphthalene (Segawa, Y.;
Yamashita, M.; Nozaki, K. Science 2006, 314, 113–115):
Ar Ar
Li,
N N
B Br B Li
THF
N − 45 °C, 6 h N
Ar Ar
(3.41)
Ar =
Note the close structural similarity of the boryllithium with an NHC. This is not a coinci-
dence: the two species are in fact isoelectronic.
In spite of its uniqueness, the above boryllithium was found to behave more or less like
a regular organolithium. Thus, it undergoes transmetallation with MgBr ⋅OEt to yield a
2
2
boryl-Grignard reagent. Similarly, with electrophiles, it reacts like a conventional carban-
ion:
Ar
N
MeOTf
B Me
N
Ar Ar Ar
N N
n-BuCI
B Li B n-Bu (3.42)
N N
Ar Ar Ar
N OH
PhCHO
B
N Ph
Ar