Page 103 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 103
3.5 LOW-OXIDATION-STATE COMPOUNDS 83
Note that this reaction is not a simple ligand substitution but a net reduction. The yields are
not particularly good, and the Ga tetrahedra are obtained more efficaciously via reaction
4
3.29, that is, the alkali metal reduction of REX derivatives.
2
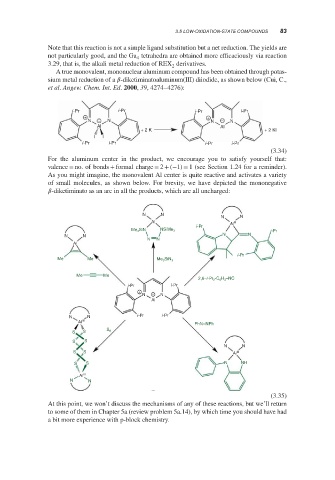
A true monovalent, mononuclear aluminum compound has been obtained through potas-
sium metal reduction of a -diketiminatoaluminum(III) diiodide, as shown below (Cui, C.,
et al. Angew. Chem. Int. Ed. 2000, 39, 4274–4276):
i-Pr i-Pr i-Pr i-Pr
+ +
N − N N − N
Al Al
+ 2 K + 2 Kl
i-Pr i-Pr i-Pr i-Pr
(3.34)
For the aluminum center in the product, we encourage you to satisfy yourself that:
valence = no. of bonds + formal charge = 2 + (−1) = 1 (see Section 1.24 for a reminder).
As you might imagine, the monovalent Al center is quite reactive and activates a variety
of small molecules, as shown below. For brevity, we have depicted the mononegative
-diketiminato as an arc in all the products, which are all uncharged:
N N
N N
Al Al III
i-Pr
Me 3 SiN NSiMe 3 i-Pr
N N N N
N N
Al
i-Pr
Me Me Me 3 SiN 3
Me Me
2,6–i-Pr 2 -C 6 H 3 –NC
i-Pr i-Pr
+
N − N
Al
N N i-Pr i-Pr
Al III
PhN=NPh
S S S 8
S S
N N
S S Al III
S S N NH
Al III
N N
(3.35)
At this point, we won’t discuss the mechanisms of any of these reactions, but we’ll return
to some of them in Chapter 5a (review problem 5a.14), by which time you should have had
a bit more experience with p-block chemistry.