Page 101 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 101
3.5 LOW-OXIDATION-STATE COMPOUNDS 81
the metals in true monovalent form. (Have a look at Section 1.24 for a reminder on the
distinction between valence and oxidation state.) A common route to low-oxidation-state
group 13 compounds consists of the reductive dehalogenation of R EX and REX (E = Al,
2 2
Ga; X = Cl, Br) with alkali metals or Rieke magnesium.
R R
− 2 KCl
2 R 2 ECl + 2 K E E (3.28)
R R
− 4 MX
REX 2 + 4 M (RE) n (3.29)
n = 1–4
For stable products, the R groups should ideally be sterically hindered aryl or alkyl groups;
examples follow in the discussion below. A typical example of an (RE) oligomer is the
n
following:
R
E
R
E
E E
R R
Note that the six “bonds” linking the vertices of the tetrahedron are not normal two-electron
bonds but are part of a delocalized bonding network; the tetrahedron is held together by
eight valence electrons, two from each group 13 element.
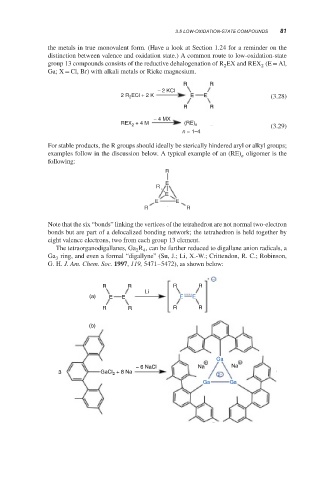
The tetraorganodigallanes, Ga R , can be further reduced to digallane anion radicals, a
2 4
Ga ring, and even a formal “digallyne” (Su, J.; Li, X.-W.; Crittendon, R. C.; Robinson,
3
G. H. J. Am. Chem. Soc. 1997, 119, 5471–5472), as shown below:
−
R R R R
Li
(a) E E E E
R R R R
(b)
Ga
+ +
− 6 NaCl Na Na
3 GaCl 2 + 8 Na 2−
Ga Ga