Page 98 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 98
GROUP 13 ELEMENTS
78
H Cl H H H
H
B Cl − Cl N Cl + Cl N Cl
Cl N − Cl + − H
B Cl B B B B
Cl Cl Cl Cl
Cl
H
Cl N Cl
B B
N N
H B H
Cl
(3.20)
REVIEW PROBLEM 3.9
A modern and relatively convenient borazine synthesis uses lithium borohydride and
ammonium chloride instead as the starting materials:
3LiBH + 3NH Cl → B H N + 3LiCl + 9H 2
4
3
3
6
4
Sketch out a mechanism for the process.
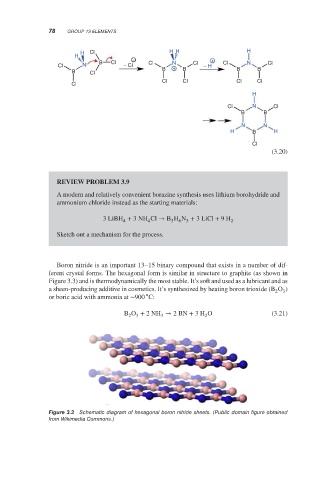
Boron nitride is an important 13–15 binary compound that exists in a number of dif-
ferent crystal forms. The hexagonal form is similar in structure to graphite (as shown in
Figure 3.3) and is thermodynamically the most stable. It’s soft and used as a lubricant and as
a sheen-producing additive in cosmetics. It’s synthesized by heating boron trioxide (B O )
3
2
∘
or boric acid with ammonia at ∼900 C:
B O + 2NH → 2BN + 3H O (3.21)
3
2
2
3
Figure 3.3 Schematic diagram of hexagonal boron nitride sheets. (Public domain figure obtained
from Wikimedia Commons.)