Page 93 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 93
3.3 GROUP 13-BASED REDUCING AGENTS 73
3.3 GROUP 13-BASED REDUCING AGENTS
−
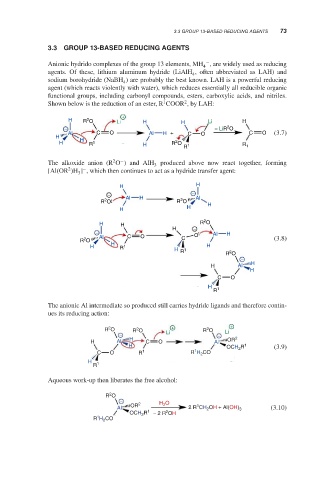
Anionic hydrido complexes of the group 13 elements, MH , are widely used as reducing
4
agents. Of these, lithium aluminum hydride (LiAlH , often abbreviated as LAH) and
4
sodium borohydride (NaBH ) are probably the best known. LAH is a powerful reducing
4
agent (which reacts violently with water), which reduces essentially all reducible organic
functional groups, including carbonyl compounds, esters, carboxylic acids, and nitriles.
1
2
Shown below is the reduction of an ester, R COOR , by LAH:
+
2
H R O Li H H Li H
2
− − LiR O
Al C O Al H + C O C O (3.7)
H
2
H H R 1 H R O R 1 R 1
−
2
The alkoxide anion (R O ) and AlH produced above now react together, forming
3
−
2
[Al(OR )H ] , which then continues to act as a hydride transfer agent:
3
H H
− Al H − Al
2
2
R O R O H
H
H
2
H H R O
H −
− O Al H
Al C O C
2
R O (3.8)
H
H R 1 H R 1 H 2
R O
−
H Al H
H
C O
H
R 1
The anionic Al intermediate so produced still carries hydride ligands and therefore contin-
ues its reducing action:
2
R O R O Li + R O Li +
2
2
− −
H Al H C O Al OR 2
H OCH 2 R 1 (3.9)
1
C O R 1 R H 2 CO
H
R 1
Aqueous work-up then liberates the free alcohol:
2
R O
− O
OR 2 H 2 1
Al 2 R CH OH + Al(OH) 3 (3.10)
2
2
OCH 2 R 1 − 2 R OH
1
R H 2 CO