Page 88 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 88
GROUP 13 ELEMENTS
68
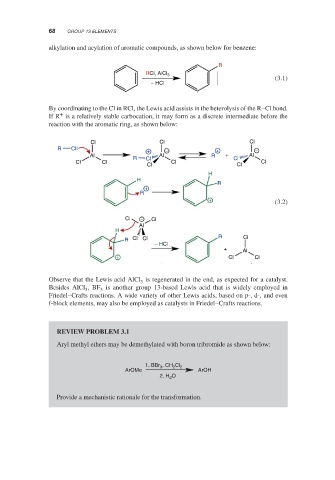
alkylation and acylation of aromatic compounds, as shown below for benzene:
R
RCl, AlCl 3
(3.1)
− HCl
By coordinating to the Cl in RCl, the Lewis acid assists in the heterolysis of the R–Cl bond.
+
If R is a relatively stable carbocation, it may form as a discrete intermediate before the
reaction with the aromatic ring, as shown below:
Cl Cl Cl
R Cl
+ − + −
Al R Al R + Cl Al
Cl Cl Cl Cl Cl
Cl Cl
H
H
R
+
R
+ (3.2)
Cl − Cl
Al
H
R Cl Cl R Cl
− HCl
+ Al
+ Cl Cl
Observe that the Lewis acid AlCl is regenerated in the end, as expected for a catalyst.
3
Besides AlCl ,BF is another group 13-based Lewis acid that is widely employed in
3 3
Friedel–Crafts reactions. A wide variety of other Lewis acids, based on p-, d-, and even
f-block elements, may also be employed as catalysts in Friedel–Crafts reactions.
REVIEW PROBLEM 3.1
Aryl methyl ethers may be demethylated with boron tribromide as shown below:
1. BBr 3 , CH 2 Cl 2
ArOMe ArOH
2. H 2 O
Provide a mechanistic rationale for the transformation.