Page 83 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 83
I
I
2.7 A Mg –Mg BOND 63
REVIEW PROBLEM 2.6
Little mechanistic information is available for the carbene-mediated dihydrogen
activation shown above. In the absence of such information, a concerted oxidative
addition seems as plausible as anything else. Sketch out such a pathway using
conventional arrow pushing.
I
I
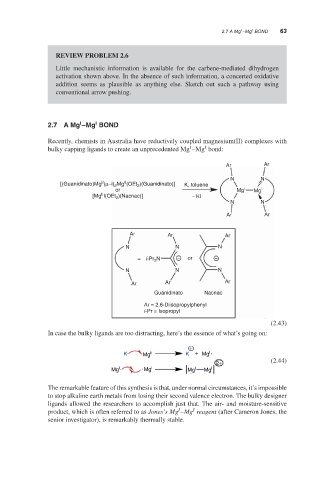
2.7 A Mg –Mg BOND
Recently, chemists in Australia have reductively coupled magnesium(II) complexes with
I
I
bulky capping ligands to create an unprecedented Mg –Mg bond:
Ar Ar
N N
II
II
[(Guanidinato)Mg (μ–l) Mg (OEt )(Guanidinato)] K, toluene
2
2
or Mg I Mg I
II
[Mg I(OEt )(Nacnac)] − KI
2
N N
Ar Ar
Ar Ar Ar
N N N
= i-Pr N − or −
2
N N N
Ar Ar Ar
Guanidinato Nacnac
Ar = 2,6-Diisopropylphenyl
i-Pr = Isopropyl
(2.43)
In case the bulky ligands are too distracting, here’s the essence of what’s going on:
+
K Mg II K + Mg I
2+ (2.44)
Mg I Mg I Mg I Mg I
The remarkable feature of this synthesis is that, under normal circumstances, it’s impossible
to stop alkaline earth metals from losing their second valence electron. The bulky designer
ligands allowed the researchers to accomplish just that. The air- and moisture-sensitive
I
I
product, which is often referred to as Jones’s Mg –Mg reagent (after Cameron Jones, the
senior investigator), is remarkably thermally stable.