Page 80 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 80
THE s-BLOCK ELEMENTS: ALKALI AND ALKALINE EARTH METALS
60
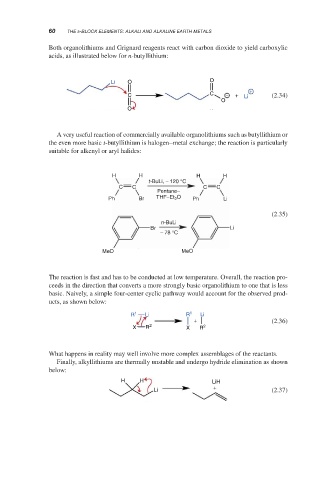
Both organolithiums and Grignard reagents react with carbon dioxide to yield carboxylic
acids, as illustrated below for n-butyllithium:
Li O O
+
C C − + Li (2.34)
O
O
A very useful reaction of commercially available organolithiums such as butyllithium or
the even more basic t-butyllithium is halogen–metal exchange; the reaction is particularly
suitable for alkenyl or aryl halides:
H H H H
t-BuLi, − 120 °C
C C C C
Pentane–
Ph Br THF–Et O Ph Li
2
(2.35)
n-BuLi
Br Li
− 78 °C
MeO MeO
The reaction is fast and has to be conducted at low temperature. Overall, the reaction pro-
ceeds in the direction that converts a more strongly basic organolithium to one that is less
basic. Naively, a simple four-center cyclic pathway would account for the observed prod-
ucts, as shown below:
R 1 Li R 1 Li
+ (2.36)
X R 2 X R 2
What happens in reality may well involve more complex assemblages of the reactants.
Finally, alkyllithiums are thermally unstable and undergo hydride elimination as shown
below:
H H LiH
+
Li (2.37)