Page 77 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 77
2.4 DISSOLVING METAL REACTIONS 57
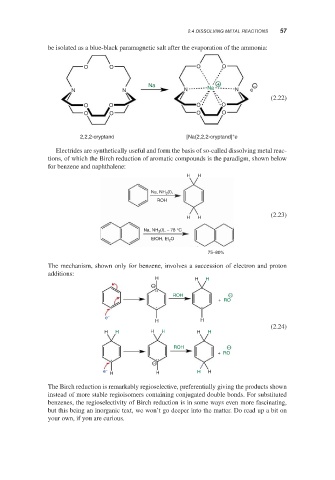
be isolated as a blue-black paramagnetic salt after the evaporation of the ammonia:
O O O O
Na Na + −
N N N N e
(2.22)
O O O O
O O O O
+ −
2,2,2-cryptand [Na(2,2,2-cryptand] e
Electrides are synthetically useful and form the basis of so-called dissolving metal reac-
tions, of which the Birch reduction of aromatic compounds is the paradigm, shown below
for benzene and naphthalene:
H H
Na, NH 3 (I),
ROH
(2.23)
H H
Na, NH 3 (I), − 78 °C
EtOH, Et 2 O
75–80%
The mechanism, shown only for benzene, involves a succession of electron and proton
additions:
H H H
−
ROH −
+ RO
−
e
H H
(2.24)
H H H H H H
ROH −
+ RO
−
e − H H
H H
The Birch reduction is remarkably regioselective, preferentially giving the products shown
instead of more stable regioisomers containing conjugated double bonds. For substituted
benzenes, the regioselectivity of Birch reduction is in some ways even more fascinating,
but this being an inorganic text, we won’t go deeper into the matter. Do read up a bit on
your own, if you are curious.