Page 76 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 76
THE s-BLOCK ELEMENTS: ALKALI AND ALKALINE EARTH METALS
56
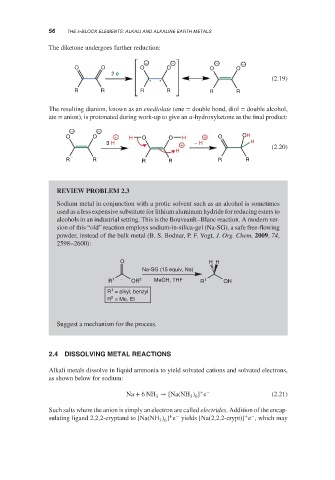
The diketone undergoes further reduction:
− − − −
O O O O O O
2 e −
(2.19)
R R R R R R
The resulting dianion, known as an enediolate (ene = double bond, diol = double alcohol,
ate = anion), is protonated during work-up to give an -hydroxyketone as the final product:
− −
O O + H O O H + O OH
3 H + − H H (2.20)
H
R R R R R R
REVIEW PROBLEM 2.3
Sodium metal in conjunction with a protic solvent such as an alcohol is sometimes
used as a less expensive substitute for lithium aluminum hydride for reducing esters to
alcohols in an industrial setting. This is the Bouveault–Blanc reaction. A modern ver-
sion of this “old” reaction employs sodium-in-silica-gel (Na-SG), a safe free-flowing
powder, instead of the bulk metal (B. S. Bodnar, P. F. Vogt, J. Org. Chem. 2009, 74,
2598–2600):
O HH
Na-SG (15 equiv, Na)
R 1 OR 2 MeOH, THF R 1 OH
1
R = alkyl, benzyl
2
R = Me, Et
Suggest a mechanism for the process.
DISSOLVING METAL REACTIONS
2.4
Alkali metals dissolve in liquid ammonia to yield solvated cations and solvated electrons,
as shown below for sodium:
+ −
Na + 6NH → [Na(NH ) ] e (2.21)
3 3 6
Such salts where the anion is simply an electron are called electrides. Addition of the encap-
+ −
+ −
sulating ligand 2,2,2-cryptand to [Na(NH ) ] e yields [Na(2,2,2-crypt)] e , which may
3 6