Page 72 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 72
THE s-BLOCK ELEMENTS: ALKALI AND ALKALINE EARTH METALS
52
O O
O O O O
O O
O O
O O O O
O
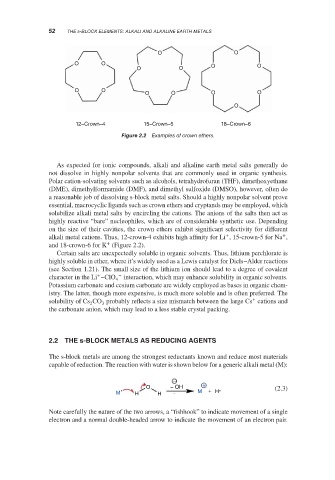
12–Crown–4 15–Crown–5 18–Crown–6
Examples of crown ethers.
Figure 2.2
As expected for ionic compounds, alkali and alkaline earth metal salts generally do
not dissolve in highly nonpolar solvents that are commonly used in organic synthesis.
Polar cation-solvating solvents such as alcohols, tetrahydrofuran (THF), dimethoxyethane
(DME), dimethylformamide (DMF), and dimethyl sulfoxide (DMSO), however, often do
a reasonable job of dissolving s-block metal salts. Should a highly nonpolar solvent prove
essential, macrocyclic ligands such as crown ethers and cryptands may be employed, which
solubilize alkali metal salts by encircling the cations. The anions of the salts then act as
highly reactive “bare” nucleophiles, which are of considerable synthetic use. Depending
on the size of their cavities, the crown ethers exhibit significant selectivity for different
+ +
alkali metal cations. Thus, 12-crown-4 exhibits high affinity for Li , 15-crown-5 for Na ,
+
and 18-crown-6 for K (Figure 2.2).
Certain salts are unexpectedly soluble in organic solvents. Thus, lithium perchlorate is
highly soluble in ether, where it’s widely used as a Lewis catalyst for Diels–Alder reactions
(see Section 1.21). The small size of the lithium ion should lead to a degree of covalent
+ −
character in the Li –ClO 4 interaction, which may enhance solubility in organic solvents.
Potassium carbonate and cesium carbonate are widely employed as bases in organic chem-
istry. The latter, though more expensive, is much more soluble and is often preferred. The
+
solubility of Cs CO probably reflects a size mismatch between the large Cs cations and
2
3
the carbonate anion, which may lead to a less stable crystal packing.
2.2 THE s-BLOCK METALS AS REDUCING AGENTS
The s-block metals are among the strongest reductants known and reduce most materials
capable of reduction. The reaction with water is shown below for a generic alkali metal (M):
−
O − OH + (2.3)
M H H M + H
Note carefully the nature of the two arrows, a “fishhook” to indicate movement of a single
electron and a normal double-headed arrow to indicate the movement of an electron pair.