Page 75 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 75
2.3 REDUCTIVE COUPLINGS 55
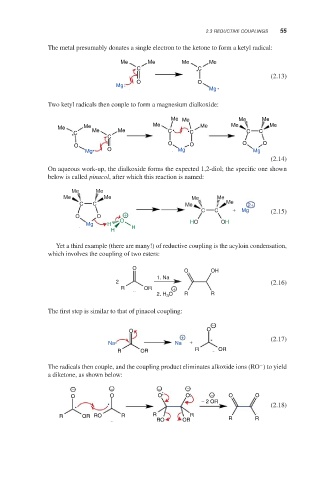
The metal presumably donates a single electron to the ketone to form a ketyl radical:
Me Me Me Me
C C
(2.13)
O O
Mg
Mg
Two ketyl radicals then couple to form a magnesium dialkoxide:
Me Me Me Me
Me Me Me Me Me Me Me Me
C C C C C
C
O O O O O
Mg O Mg Mg
(2.14)
On aqueous work-up, the dialkoxide forms the expected 1,2-diol; the specific one shown
below is called pinacol, after which this reaction is named:
Me Me
Me Me Me Me
C C Me Me 2+
C C + Mg (2.15)
O O +
O HO OH
Mg H
H H
Yet a third example (there are many!) of reductive coupling is the acyloin condensation,
which involves the coupling of two esters:
O
O OH
1. Na
2 (2.16)
R OR +
2. H 3 O R R
The first step is similar to that of pinacol coupling:
−
O O
+ (2.17)
Na Na +
R OR R OR
−
The radicals then couple, and the coupling product eliminates alkoxide ions (RO ) to yield
a diketone, as shown below:
− − − −
O O O O − O O
− 2 OR
(2.18)
R OR RO R R R
RO OR R R