Page 82 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 82
THE s-BLOCK ELEMENTS: ALKALI AND ALKALINE EARTH METALS
62
Even more excitingly, the above zwitterion catalyzes the hydrogenation of polar
carbon–carbon double bonds, traditionally a bastion of transition-metal catalysts. The
following example involves an enamine, an uncharged nitrogen equivalent of an enolate
anion (RT = room temperature):
H
+
Mes P B(C F )
6 5 2
−
2
N H (5 mol%) N
(2.40)
RT, 1.5 atm. H
2
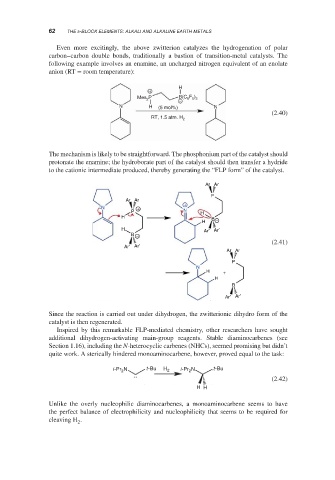
The mechanism is likely to be straightforward. The phosphonium part of the catalyst should
protonate the enamine; the hydroborate part of the catalyst should then transfer a hydride
to the cationic intermediate produced, thereby generating the “FLP form” of the catalyst.
Ar Ar
P
Ar Ar
+
N +
P N
H
H B
H −
H Ar′ Ar′
B −
(2.41)
Ar′ Ar′
Ar Ar
P
N
H +
H
B
Ar′ Ar′
Since the reaction is carried out under dihydrogen, the zwitterionic dihydro form of the
catalyst is then regenerated.
Inspired by this remarkable FLP-mediated chemistry, other researchers have sought
additional dihydrogen-activating main-group reagents. Stable diaminocarbenes (see
Section 1.16), including the N-heterocyclic carbenes (NHCs), seemed promising but didn’t
quite work. A sterically hindered monoaminocarbene, however, proved equal to the task:
i-Pr N t-Bu H 2 i-Pr N t-Bu
2
2
(2.42)
H H
Unlike the overly nucleophilic diaminocarbenes, a monoaminocarbene seems to have
the perfect balance of electrophilicity and nucleophilicity that seems to be required for
cleaving H .
2