Page 84 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 84
THE s-BLOCK ELEMENTS: ALKALI AND ALKALINE EARTH METALS
64
REVIEW PROBLEM 2.7
Comment on the magnesium oxidation state, valence, and coordination number in
Jones’s reagent.
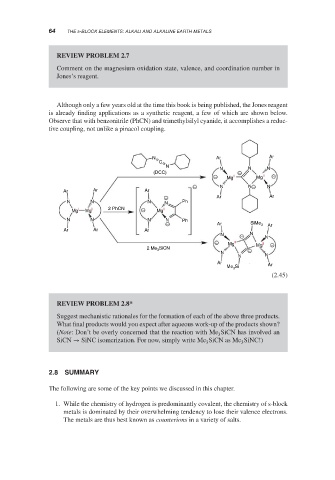
Although only a few years old at the time this book is being published, the Jones reagent
is already finding applications as a synthetic reagent, a few of which are shown below.
Observe that with benzonitrile (PhCN) and trimethylsilyl cyanide, it accomplishes a reduc-
tive coupling, not unlike a pinacol coupling.
N Ar Ar
C
N
N N N
(DCC) −
− Mg II Mg II −
− N N − N
Ar Ar Ar
− Ar Ar
N N N N Ph
Mg I Mg I 2 PhCN − Mg II
N N N N Ph
− Ar SiMe 3 Ar
Ar Ar Ar
N − N N
− Mg II II
2 Me SiCN Mg −
3 −
N
N N
Ar Ar
Me Si
3
(2.45)
REVIEW PROBLEM 2.8*
Suggest mechanistic rationales for the formation of each of the above three products.
What final products would you expect after aqueous work-up of the products shown?
(Note: Don’t be overly concerned that the reaction with Me SiCN has involved an
3
SiCN → SiNC isomerization. For now, simply write Me SiCN as Me SiNC!)
3 3
2.8 SUMMARY
The following are some of the key points we discussed in this chapter.
1. While the chemistry of hydrogen is predominantly covalent, the chemistry of s-block
metals is dominated by their overwhelming tendency to lose their valence electrons.
The metals are thus best known as counterions in a variety of salts.