Page 91 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 91
3.2 HYDROBORATION 71
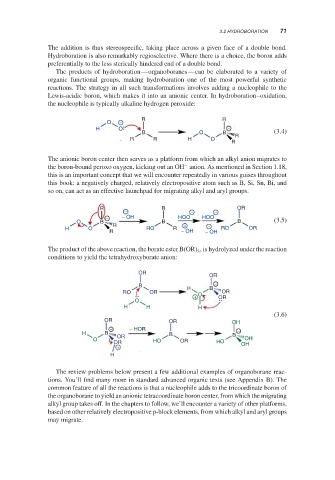
The addition is thus stereospecific, taking place across a given face of a double bond.
Hydroboration is also remarkably regioselective. Where there is a choice, the boron adds
preferentially to the less sterically hindered end of a double bond.
The products of hydroboration—organoboranes—can be elaborated to a variety of
organic functional groups, making hydroboration one of the most powerful synthetic
reactions. The strategy in all such transformations involves adding a nucleophile to the
Lewis-acidic boron, which makes it into an anionic center. In hydroboration–oxidation,
the nucleophile is typically alkaline hydrogen peroxide:
R R
O −
H O −
B O B (3.4)
R
R R H O
R
The anionic boron center then serves as a platform from which an alkyl anion migrates to
−
the boron-bound peroxo oxygen, kicking out an OH anion. As mentioned in Section 1.18,
this is an important concept that we will encounter repeatedly in various guises throughout
this book: a negatively charged, relatively electropositive atom such as B, Si, Sn, Bi, and
so on, can act as an effective launchpad for migrating alkyl and aryl groups.
R R OR
− − −
− − OH HOO HOO
O B B B (3.5)
R − −
H O RO R RO OR
R − OH − OH
The product of the above reaction, the borate ester B(OR) , is hydrolyzed under the reaction
3
conditions to yield the tetrahydroxyborate anion:
OR
OR
−
B H B
RO OR O OR
+ OR
O
H H H
(3.6)
OR OR OH
− − HOR
H B B −
OR B
O HO OR OH
OR HO OH
+
H
The review problems below present a few additional examples of organoborane reac-
tions. You’ll find many more in standard advanced organic texts (see Appendix B). The
common feature of all the reactions is that a nucleophile adds to the tricoordinate boron of
the organoborane to yield an anionic tetracoordinate boron center, from which the migrating
alkyl group takes off. In the chapters to follow, we’ll encounter a variety of other platforms,
based on other relatively electropositive p-block elements, from which alkyl and aryl groups
may migrate.