Page 96 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 96
GROUP 13 ELEMENTS
76
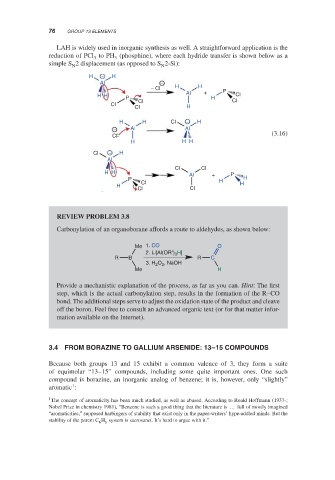
LAH is widely used in inorganic synthesis as well. A straightforward application is the
reduction of PCl to PH (phosphine), where each hydride transfer is shown below as a
3 3
simple S 2 displacement (as opposed to S 2-Si):
N N
H − H
Al −
− Cl H H
Al + P
H H Cl
P H
Cl Cl
Cl
Cl H
H H Cl − H
− Al Al
Cl (3.16)
H HH
Cl − H
Al
Cl Cl
H H
Al + P
P H H
Cl H
H
Cl Cl
REVIEW PROBLEM 3.8
Carbonylation of an organoborane affords a route to aldehydes, as shown below:
Me 1. CO O
2. Li[Al(OR′) 3 H]
R B R C
3. H 2 O 2 , NaOH
Me H
Provide a mechanistic explanation of the process, as far as you can. Hint: The first
step, which is the actual carbonylation step, results in the formation of the R–CO
bond. The additional steps serve to adjust the oxidation state of the product and cleave
off the boron. Feel free to consult an advanced organic text (or for that matter infor-
mation available on the Internet).
FROM BORAZINE TO GALLIUM ARSENIDE: 13–15 COMPOUNDS
3.4
Because both groups 13 and 15 exhibit a common valence of 3, they form a suite
of equimolar “13–15” compounds, including some quite important ones. One such
compound is borazine, an inorganic analog of benzene; it is, however, only “slightly”
1
aromatic :
1 The concept of aromaticity has been much studied, as well as abused. According to Roald Hoffmann (1937-;
Nobel Prize in chemistry 1981), “Benzene is such a good thing that the literature is … full of mostly imagined
“aromaticities,” supposed harbingers of stability that exist only in the paper-writers’ hype-addled minds. But the
stability of the parent C H system is sacrosanct. It’s hard to argue with it.”
6
6