Page 97 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 97
3.4 FROM BORAZINE TO GALLIUM ARSENIDE: 13–15 COMPOUNDS 77
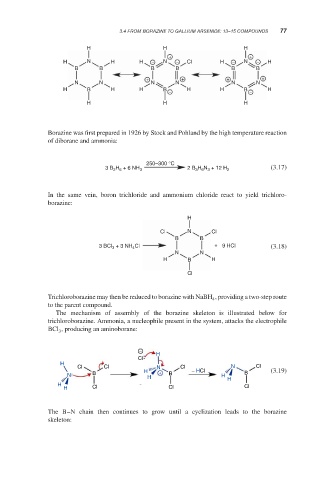
H H H
+ +
H N H H − N − Cl H − N − H
B B B B B B
+ + + +
N N N N N N
H B H H B − H H B − H
H H H
Borazine was first prepared in 1926 by Stock and Pohland by the high temperature reaction
of diborane and ammonia:
250–300 °C
3 B H + 6 NH 3 2 B H N + 12 H 2 (3.17)
3 6 3
2 6
In the same vein, boron trichloride and ammonium chloride react to yield trichloro-
borazine:
H
Cl N Cl
B B
3 BCl 3 + 3 NH 4 Cl + 9 HCl (3.18)
N N
H B H
Cl
Trichloroborazine may then be reduced to borazine with NaBH , providing a two-step route
4
to the parent compound.
The mechanism of assembly of the borazine skeleton is illustrated below for
trichloroborazine. Ammonia, a nucleophile present in the system, attacks the electrophile
BCl , producing an aminoborane:
3
−
H
Cl
H
Cl Cl N Cl N Cl
N B H + B − HCl H B (3.19)
H H
H Cl
H Cl Cl
The B–N chain then continues to grow until a cyclization leads to the borazine
skeleton: