Page 100 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 100
GROUP 13 ELEMENTS
80
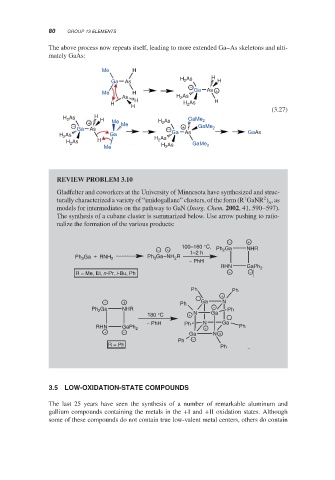
The above process now repeats itself, leading to more extended Ga–As skeletons and ulti-
mately GaAs:
Me H
H As H
Ga As 2 H
−
Me H Ga As +
As H 2 As
H H
H H As
2
H
(3.27)
H As H H GaMe
2
+ Me Me H 2 As 2
− + GaMe
Ga As − 2
H 2 As Ga H As Ga As GaAs
H As H 2
2
Me H As GaMe 2
2
REVIEW PROBLEM 3.10
Gladfelter and coworkers at the University of Minnesota have synthesized and struc-
2
1
turally characterized a variety of “imidogallane” clusters, of the form (R GaNR ) ,as
n
models for intermediates on the pathway to GaN (Inorg. Chem. 2002, 41, 590–597).
The synthesis of a cubane cluster is summarized below. Use arrow pushing to ratio-
nalize the formation of the various products:
− +
100–160 °C,
− + Ph 2 Ga NHR
Ph Ga NH 2 R 1–2 h
Ph 3 Ga + RNH 2 3
− PhH
RHN GaPh 2
R = Me, Et, n-Pr, i-Bu, Ph + −
Ph Ph
− +
− + Ph Ga N
Ph Ga NHR − Ph
2
180 °C + N Ga −
− PhH Ph N Ga
RHN GaPh 2 + Ph
+ − Ga N +
Ph −
R = Ph Ph
3.5 LOW-OXIDATION-STATE COMPOUNDS
The last 25 years have seen the synthesis of a number of remarkable aluminum and
gallium compounds containing the metals in the +I and +II oxidation states. Although
some of these compounds do not contain true low-valent metal centers, others do contain