Page 99 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 99
3.4 FROM BORAZINE TO GALLIUM ARSENIDE: 13–15 COMPOUNDS 79
B(OH) + NH → BN + 3H O (3.22)
3
2
3
An exciting recent development has been the preparation of single sheets of hexagonal
BN, dubbed “white graphene” by analogy with graphene, which refers to single sheets of
graphite. These are found to soak up prodigious quantities of various pollutants out of water,
such as ethylene glycol (antifreeze) and engine oil. Impressively, the pollutants could then
be driven out by heating in a furnace, and the boron nitride could be recovered and reused.
Cubic boron nitride, on the other hand, has the same structure as diamond but is inferior to
diamond only in hardness. Like diamond, it’s widely used as an abrasive.
Gallium nitride and gallium arsenide are the preeminent examples of 13–15 semiconduc-
tors. The 13–15 semiconductors are overall isoelectronic with silicon and greatly expand
the repertoire of available, useful properties relative to the elemental silicon and germanium.
Gallium arsenide is used in the manufacture of a wide range of integrated circuits, laser
diodes, laser lenses, reflectors, and solar cells. Gallium nitride, widely used as light-emitting
diodes since the 1990s, is considered even more exciting. Its high thermal stability com-
pared with GaAs makes it an ideal material for high power transistors capable of operating
at high temperatures. Several preparative routes are known for both materials. For our
mechanistic discussion, we will focus on GaAs synthesis via a specialized technique called
metal-organic vapor phase epitaxy (MOVPE) or metal-organic chemical vapor deposition
(MOCVD):
Ga(CH ) + AsH → GaAs + 3CH 4 (3.23)
3
3 3
The reactants, in the form of ultrapure gases, are injected into a reactor and dosed so as to
deposit a thin layer on a semiconductor wafer.
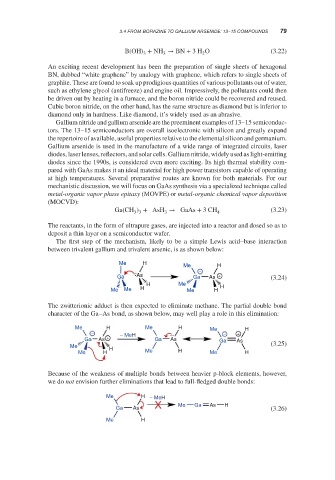
The first step of the mechanism, likely to be a simple Lewis acid–base interaction
between trivalent gallium and trivalent arsenic, is as shown below:
Me H
Me H
−
Ga As Ga As + (3.24)
H Me
Me Me H Me H H
The zwitterionic adduct is then expected to eliminate methane. The partial double bond
character of the Ga–As bond, as shown below, may well play a role in this elimination:
Me H Me H Me H
− − MeH − +
Ga As + Ga As Ga As
Me H (3.25)
Me H Me H Me H
Because of the weakness of multiple bonds between heavier p-block elements, however,
we do not envision further eliminations that lead to full-fledged double bonds:
Me H − MeH
Me Ga As H
Ga As (3.26)
Me H