Page 94 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 94
GROUP 13 ELEMENTS
74
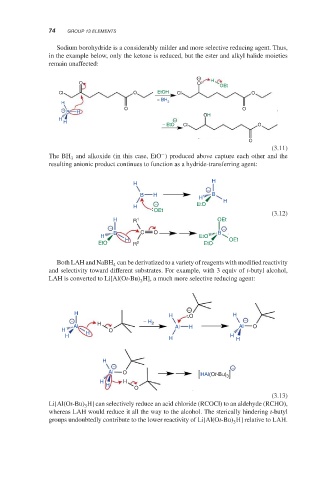
Sodium borohydride is a considerably milder and more selective reducing agent. Thus,
in the example below, only the ketone is reduced, but the ester and alkyl halide moieties
remain unaffected:
− H
O O
OEt
Cl O EtOH Cl O
H − BH 3
− B H O O
OH
H −
H
− EtO Cl O
O
(3.11)
−
The BH and alkoxide (in this case, EtO ) produced above capture each other and the
3
resulting anionic product continues to function as a hydride-transferring agent:
H
H
−
B H B
H
− EtO H
H
OEt
(3.12)
H R 1 OEt
− −
B C O B
H EtO
H OEt
EtO R 2 EtO
Both LAH and NaBH can be derivatized to a variety of reagents with modified reactivity
4
and selectivity toward different substrates. For example, with 3 equiv of t-butyl alcohol,
LAH is converted to Li[Al(Ot-Bu) H], a much more selective reducing agent:
3
−
H H O H
− H − H 2 −
Al Al H Al O
H H O
H H H H
H
− −
Al O
HAl(Ot-Bu) 3
H H
H
O
(3.13)
Li[Al(Ot-Bu) H] can selectively reduce an acid chloride (RCOCl) to an aldehyde (RCHO),
3
whereas LAH would reduce it all the way to the alcohol. The sterically hindering t-butyl
groups undoubtedly contribute to the lower reactivity of Li[Al(Ot-Bu) H] relative to LAH.
3