Page 95 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 95
3.3 GROUP 13-BASED REDUCING AGENTS 75
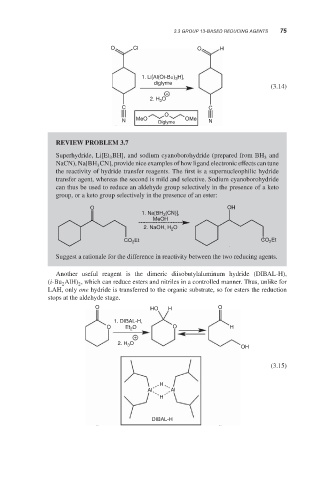
O Cl O H
1. Li[Al(Ot-Bu) 3 H],
diglyme
(3.14)
+
2. H 3 O
C C
O
N MeO Diglyme OMe N
REVIEW PROBLEM 3.7
Superhydride, Li[Et BH], and sodium cyanoborohydride (prepared from BH and
3
3
NaCN), Na[BH CN], provide nice examples of how ligand electronic effects can tune
3
the reactivity of hydride transfer reagents. The first is a supernucleophilic hydride
transfer agent, whereas the second is mild and selective. Sodium cyanoborohydride
can thus be used to reduce an aldehyde group selectively in the presence of a keto
group, or a keto group selectively in the presence of an ester:
O OH
(CN)],
1. Na[BH 3
MeOH
2. NaOH, H 2 O
CO Et CO 2 Et
2
Suggest a rationale for the difference in reactivity between the two reducing agents.
Another useful reagent is the dimeric diisobutylaluminum hydride (DIBAL-H),
(i-Bu AlH) , which can reduce esters and nitriles in a controlled manner. Thus, unlike for
2
2
LAH, only one hydride is transferred to the organic substrate, so for esters the reduction
stops at the aldehyde stage.
O HO H O
1. DIBAL-H,
O Et O O H
2
+
O
2. H 3
OH
(3.15)
H
Al Al
H
DIBAL-H