Page 104 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 104
GROUP 13 ELEMENTS
84
In 1989, in a major development, Tacke and Schnöckel showed that metastable solutions
of Al and Ga monohalides could be prepared in organic solvents (Tacke, M.; Schnöckel,
H. Inorg. Chem. 1989, 28, 2895–2896). The monohalides were prepared from the molten
metal and a hydrogen halide in a high temperature reactor and subsequently condensed at
∘
−196 C with toluene, with various Lewis-base additives.
1000 °C
2 AI(g) + 2 HCI(g) 2 AICI(g) + H 2 (g) (3.36)
< 0.2 mbar
∘
On melting at approximately −100 C, the condensates yield deep red solutions of the
metal monohalides, which serve as excellent starting materials for a variety of low
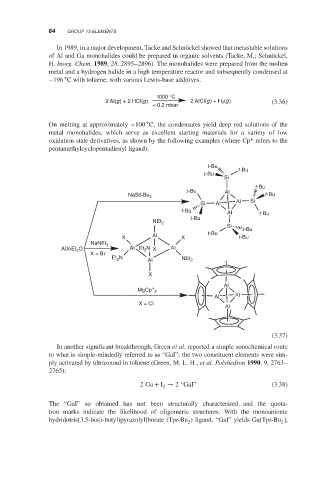
oxidation-state derivatives, as shown by the following examples (where Cp* refers to the
pentamethylcyclopentadienyl ligand):
t-Bu
t-Bu
t-Bu
Si
t-Bu
t-Bu AI
NaSit-Bu 3 t-Bu
AI Si
Si AI
t-Bu AI t-Bu
t-Bu
NEt 2
Si
t-Bu
X AI X t-Bu t-Bu
NaNEt 2
AIX•Et 2 O AI Et 2 N X AI
X = Br
Et N AI NEt 2
2
X
∗ AI
MgCp 2
AI AI
X = CI
AI
(3.37)
In another significant breakthrough, Green et al. reported a simple sonochemical route
to what is simple-mindedly referred to as “GaI”; the two constituent elements were sim-
ply activated by ultrasound in toluene (Green, M. L. H., et al. Polyhedron 1990, 9, 2763–
2765):
2Ga + I → 2 “GaI” (3.38)
2
The “GaI” so obtained has not been structurally characterized and the quota-
tion marks indicate the likelihood of oligomeric structures. With the monoanionic
hydridotris[3,5-bis(t-butyl)pyrazolyl]borate (Tpt-Bu ) ligand, “GaI” yields Ga[Tpt-Bu ],
2
2