Page 106 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 106
GROUP 13 ELEMENTS
86
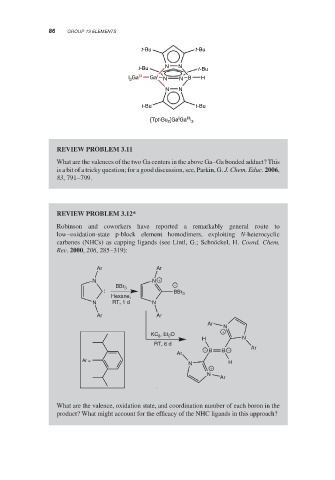
t-Bu t-Bu
t-Bu N N t-Bu
l 3 Ga III Ga I N N B H
N N
t-Bu t-Bu
I III
[Tpt-Bu 2 ]Ga Ga l 3
REVIEW PROBLEM 3.11
What are the valences of the two Ga centers in the above Ga–Ga bonded adduct? This
is a bit of a tricky question; for a good discussion, see, Parkin, G. J. Chem. Educ. 2006,
83, 791–799.
REVIEW PROBLEM 3.12*
Robinson and coworkers have reported a remarkably general route to
low–oxidation-state p-block element homodimers, exploiting N-heterocyclic
carbenes (NHCs) as capping ligands (see Lintl, G.; Schnöckel, H. Coord. Chem.
Rev. 2000, 206, 285–319):
Ar Ar
N N +
BBr 3 −
BBr 3
Hexane,
N RT, 1 d N
Ar Ar
Ar
N
+
, Et O
KC 8 2
H N
RT, 6 d
− B B − Ar
Ar
Ar =
N H
+
N
Ar
What are the valence, oxidation state, and coordination number of each boron in the
product? What might account for the efficacy of the NHC ligands in this approach?