Page 142 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 142
GROUP 14 ELEMENTS
122
4.10 SILYL CATIONS
Given that silicon is more electropositive than carbon, it may be surprising that silyl anions
are much more stable than the analogous carbanions, whereas silyl cations are less stable
than the analogous carbocations. Multiple factors account for these differences. As men-
tioned, the stability of silyl anions reflects the relative stability of the 3s electron pair,
relative to the 3p electrons; the 3s–3p energy gap is significantly higher than the 2s–2p
gap. The lone pair of a silyl anion thus has substantial 3s character. This is essentially
the same reason why the divalent state becomes progressively more stable as one goes
down the group. The instability of silyl cations, on the other hand, may have more of
a geometric origin. The longer bonds to silicon, relative to carbon, make them extraor-
dinarily reactive as Lewis acids. Nevertheless, three sterically hindered aryl groups such
as mesityl (i.e., 2,4,6-trimethylphenyl) or duryl (i.e., 2,3,5,6-tetramethylphenyl) are suffi-
cient for generating what may be termed a moderately stable silyl cation. Very recently,
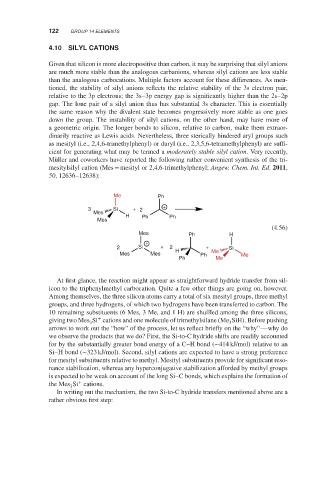
Müller and coworkers have reported the following rather convenient synthesis of the tri-
mesitylsilyl cation (Mes = mesityl or 2,4,6-trimethylphenyl; Angew. Chem. Int. Ed. 2011,
50, 12636–12638):
Me Ph
+
3 Si + 2
Mes
H Ph Ph
Mes
(4.56)
Mes Ph H
+
2 Si + 2 + Si
Mes Mes H Ph Me Me
Ph Me
At first glance, the reaction might appear as straightforward hydride transfer from sil-
icon to the triphenylmethyl carbocation. Quite a few other things are going on, however.
Among themselves, the three silicon atoms carry a total of six mesityl groups, three methyl
groups, and three hydrogens, of which two hydrogens have been transferred to carbon. The
10 remaining substituents (6 Mes, 3 Me, and 1 H) are shuffled among the three silicons,
+
giving two Mes Si cations and one molecule of trimethylsilane (Me SiH). Before pushing
3
3
arrows to work out the “how” of the process, let us reflect briefly on the “why”—why do
we observe the products that we do? First, the Si-to-C hydride shifts are readily accounted
for by the substantially greater bond energy of a C–H bond (∼414 kJ/mol) relative to an
Si–H bond (∼323 kJ/mol). Second, silyl cations are expected to have a strong preference
for mesityl substituents relative to methyl. Mesityl substituents provide for significant reso-
nance stabilization, whereas any hyperconjugative stabilization afforded by methyl groups
is expected to be weak on account of the long Si–C bonds, which explains the formation of
+
the Mes Si cations.
3
In writing out the mechanism, the two Si-to-C hydride transfers mentioned above are a
rather obvious first step: