Page 115 - Assurance of Sterility for Sensitive Combination Products and Materials
P. 115
Package/container closures 99
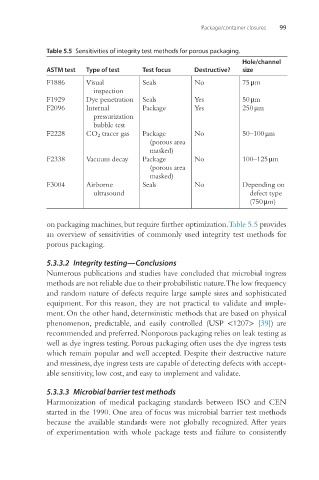
Table 5.5 Sensitivities of integrity test methods for porous packaging.
Hole/channel
ASTM test Type of test Test focus Destructive? size
F1886 Visual Seals No 75 μm
inspection
F1929 Dye penetration Seals Yes 50 μm
F2096 Internal Package Yes 250 μm
pressurization
bubble test
F2228 CO 2 tracer gas Package No 50–100 μm
(porous area
masked)
F2338 Vacuum decay Package No 100–125 μm
(porous area
masked)
F3004 Airborne Seals No Depending on
ultrasound defect type
(750 μm)
on packaging machines, but require further optimization. Table 5.5 provides
an overview of sensitivities of commonly used integrity test methods for
porous packaging.
5.3.3.2 Integrity testing—Conclusions
Numerous publications and studies have concluded that microbial ingress
methods are not reliable due to their probabilistic nature. The low frequency
and random nature of defects require large sample sizes and sophisticated
equipment. For this reason, they are not practical to validate and imple-
ment. On the other hand, deterministic methods that are based on physical
phenomenon, predictable, and easily controlled (USP <1207> [39]) are
recommended and preferred. Nonporous packaging relies on leak testing as
well as dye ingress testing. Porous packaging often uses the dye ingress tests
which remain popular and well accepted. Despite their destructive nature
and messiness, dye ingress tests are capable of detecting defects with accept-
able sensitivity, low cost, and easy to implement and validate.
5.3.3.3 Microbial barrier test methods
Harmonization of medical packaging standards between ISO and CEN
started in the 1990. One area of focus was microbial barrier test methods
because the available standards were not globally recognized. After years
of experimentation with whole package tests and failure to consistently