Page 216 - Assurance of Sterility for Sensitive Combination Products and Materials
P. 216
Risk to the patient—Quantifying assurance of sterility 195
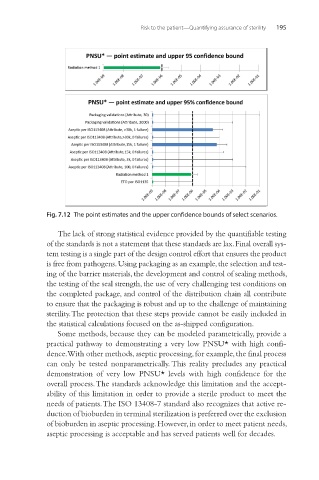
Fig. 7.12 The point estimates and the upper confidence bounds of select scenarios.
The lack of strong statistical evidence provided by the quantifiable testing
of the standards is not a statement that these standards are lax. Final overall sys-
tem testing is a single part of the design control effort that ensures the product
is free from pathogens. Using packaging as an example, the selection and test-
ing of the barrier materials, the development and control of sealing methods,
the testing of the seal strength, the use of very challenging test conditions on
the completed package, and control of the distribution chain all contribute
to ensure that the packaging is robust and up to the challenge of maintaining
sterility. The protection that these steps provide cannot be easily included in
the statistical calculations focused on the as-shipped configuration.
Some methods, because they can be modeled parametrically, provide a
practical pathway to demonstrating a very low PNSU* with high confi-
dence. With other methods, aseptic processing, for example, the final process
can only be tested nonparametrically. This reality precludes any practical
demonstration of very low PNSU* levels with high confidence for the
overall process. The standards acknowledge this limitation and the accept-
ability of this limitation in order to provide a sterile product to meet the
needs of patients. The ISO 13408-7 standard also recognizes that active re-
duction of bioburden in terminal sterilization is preferred over the exclusion
of bioburden in aseptic processing. However, in order to meet patient needs,
aseptic processing is acceptable and has served patients well for decades.