Page 217 - Assurance of Sterility for Sensitive Combination Products and Materials
P. 217
196 Assurance of sterility for sensitive combination products and materials
The discussion below related to patient risk picks-up from clause 7.1.
The intervening discussion of the statistics of quantifiable aspects of sterility
assurance grounds the following patient risk discussion and ensures a focus
on the patient and patient risk, as opposed to peripheral or nonvalue-added
sterility-related issues or paradigms in any given industry sector.
7.4 Relative patient risks from various industry sectors
compared to real-world infection rates
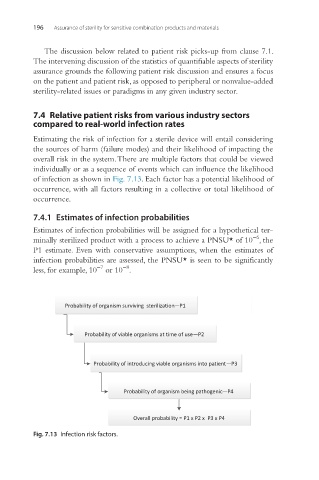
Estimating the risk of infection for a sterile device will entail considering
the sources of harm (failure modes) and their likelihood of impacting the
overall risk in the system. There are multiple factors that could be viewed
individually or as a sequence of events which can influence the likelihood
of infection as shown in Fig. 7.13. Each factor has a potential likelihood of
occurrence, with all factors resulting in a collective or total likelihood of
occurrence.
7.4.1 Estimates of infection probabilities
Estimates of infection probabilities will be assigned for a hypothetical ter-
−6
minally sterilized product with a process to achieve a PNSU* of 10 , the
P1 estimate. Even with conservative assumptions, when the estimates of
infection probabilities are assessed, the PNSU* is seen to be significantly
−8
−7
less, for example, 10 or 10 .
Fig. 7.13 Infection risk factors.