Page 213 - Assurance of Sterility for Sensitive Combination Products and Materials
P. 213
192 Assurance of sterility for sensitive combination products and materials
Start
Inoculate the device at a worst 3 successful
case location with a population inactivations in 3
6
of at least 10 Bl attempts Yes
Place device within the sterile
load as appropriate
Establish a sterilization process
with at least twice the time used
Expose the sterile load to at
most ½ the minimum exposure
time
(B) End
Verify total inactivation of the Bl
(A)
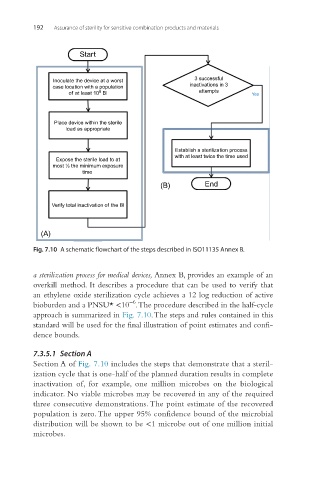
Fig. 7.10 A schematic flowchart of the steps described in ISO11135 Annex B.
a sterilization process for medical devices, Annex B, provides an example of an
overkill method. It describes a procedure that can be used to verify that
an ethylene oxide sterilization cycle achieves a 12 log reduction of active
−6
bioburden and a PNSU* <10 . The procedure described in the half-cycle
approach is summarized in Fig. 7.10. The steps and rules contained in this
standard will be used for the final illustration of point estimates and confi-
dence bounds.
7.3.5.1 Section A
Section A of Fig. 7.10 includes the steps that demonstrate that a steril-
ization cycle that is one-half of the planned duration results in complete
inactivation of, for example, one million microbes on the biological
indicator. No viable microbes may be recovered in any of the required
three consecutive demonstrations. The point estimate of the recovered
population is zero. The upper 95% confidence bound of the microbial
distribution will be shown to be <1 microbe out of one million initial
microbes.