Page 129 - Basic Gas Chromatography
P. 129
115 From New York, af Miller, 1987,
Perkin-Elmer. Inc., i 700 From York, New
of Sons, & a i 600 Perkin-Elmer. Inc., Sons,
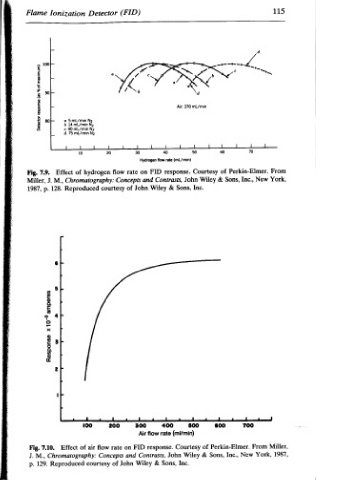
Air. 370 mL/min Courtesy : . Wiley John Inc. Sons, = 4 i $00 (mi/min) of Courtesy & Wiley Inc.
Hydrogen flow rate (mL/min) response FID Contrasts, & Wiley 1 + 400 rate Air flow response. John Contrasts, Sons, &
(FID) on rate flow and Concepts John of i . 300 FID on rate and Wiley John
Detector hydrogen Chromatography: courtesy i . i 200 100 fiow air Concepts of courtesy
Ionization i a. 5 mL/min No 6.14 mU/min No ¢. 40 mL/min No d.75 ml/min No of Effect M., Reproduced 128. 1 of Effect Chromatography: Reproduced
|
L
3
&
Flame 3 (unuuprew jo % S#) asuodsau 20;99}8g 7.9. Fig. i J. Miller, p. 1987, saiodwe g-O1X esuodsey 7.10. Fig. M., J. 129. p.
Detectors ECN Preston mL/ not significant tailed for the in water, Its but
Theoretical 2 2 6 0.5 15 2.5 2.5 3.5 45 MOF AHKAMNOEAMINAMHOCORM of 400 to are and badly used be interfere in the FID. ruggedness, phases
[15]* permission 300 of 7.10. burn Most 7.3. produces to FID not of simplicity, glycol liquid
heptane) Science by value Figure not Table the does organic contaminants products.
to and a in do in often permits it characteristics linearity, polyethylene
(relative ECN 1.95 2.00 5.82 0.52 1.48 2.52 2.24 3.42 4,37 3.12 6.14 6.99 8.73 1.01 2.07 2.95 5.11 5.55 6.55 1.04 2.33 3.52 4.46 2.00 3.07 4.97 5.66 7.15 7.16 4.94 Chromatographic Inc. critical, shown carbon listed that since include food the and
Numbers Industries, less as organic ones are compound water for water and summarizes large a columns. polyester polymers.
Carbon of Journal Preston much detectors, containing important a water, peak a contain applications beverages, sensitivity, sizes of the of silicone
Effective the of of is air most for not is of that Typical alcoholic 116 page good all to some on
FID ketone ketone ketone ketone from Division rate most listed absence samples other on are hydrolyze effect
7.2 Compound alcohol aldehyde acid acid acid acid acid acid acetate acetate acetate acetate ethyl i-butyl butyl ketone amyl Reproduced A flow sufficient Compounds The those The of chromatogram‘. and list adaptability may no to
114 TABLE Acetylene Ethylene Hexene Methanol Ethanol n-Propanol i-Propanol n-Butanol Amyl Butanal Heptanal Octanal Capric Acetic Propionic Butyric Hexanoic Heptanoic Octanoic Methyl Ethyl i-Propyl n-Butyl Acetone Methyl Methyl Ethyl Di-i-butyl Ethyl Cyclohexanone * Publications, The min is detected. among peaks. analysis wine The advantages and “Water it